Arsenite Inhibits Tissue-Type Plasminogen Activator Synthesis through NRF2 Activation in Cultured Human Vascular Endothelial EA.hy926 Cells
- PMID: 33451022
- PMCID: PMC7828481
- DOI: 10.3390/ijms22020739
Arsenite Inhibits Tissue-Type Plasminogen Activator Synthesis through NRF2 Activation in Cultured Human Vascular Endothelial EA.hy926 Cells
Abstract
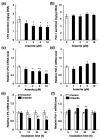
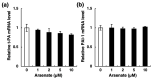
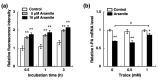
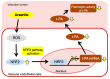
Chronic arsenic exposure is known to be related to the progression of atherosclerosis. However, the pathogenic mechanisms of arsenic-induced atherosclerosis have not been fully elucidated. Because disruption of the blood coagulation/fibrinolytic system is involved in the development of arteriosclerosis, we investigated the effect of arsenite on fibrinolytic activity in human vascular endothelial EA.hy926 cells in the present study. Fibrinolysis depends on the balance between tissue-type plasminogen activator (t-PA) and plasminogen activator inhibitor 1 (PAI-1) secreted from vascular endothelial cells. We found that arsenite reduced fibrinolytic t-PA activity by inhibiting its synthesis without affecting PAI-1 production. The inhibitory effect of arsenite on t-PA expression was partially recovered by the reactive oxygen species (ROS) scavenger Trolox. The nuclear factor erythroid 2 related factor 2 (NRF2) pathway is known to be activated by arsenite via ROS production. We confirmed that arsenite activated the NRF2 pathway, and arsenite-induced inhibition of fibrinolytic t-PA activity was abrogated in NRF2-knockdown EA.hy926 cells. These results suggest that arsenite inhibits the fibrinolytic activity of t-PA by selectively suppressing its synthesis via activation of the NRF2 pathway in vascular endothelial cells.
Keywords: arsenite; atherosclerosis; endothelial cell; fibrinolysis; nuclear factor erythroid 2 related factor 2; tissue-type plasminogen activator.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Savage K.S., Tingle T.N., O’Day P.A., Waychunas G.A., Bird D.K. Arsenic speciation in pyrite and secondary weathering phases, Mother Lode Gold District, Tuolumne County, California. Appl. Geochem. 2000;15:1219–1244. doi: 10.1016/S0883-2927(99)00115-8. - DOI
-
- Tseng W.P., Chu H.M., How S.W., Fong J.M., Lin C.S., Yeh S. Prevalence of skin cancer in an endemic area of chronic arsenicism in Taiwan. J. Natl. Cancer Inst. 1968;40:453–463. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

