The Interplay between Angiopoietin-Like Proteins and Adipose Tissue: Another Piece of the Relationship between Adiposopathy and Cardiometabolic Diseases?
- PMID: 33451033
- PMCID: PMC7828552
- DOI: 10.3390/ijms22020742
The Interplay between Angiopoietin-Like Proteins and Adipose Tissue: Another Piece of the Relationship between Adiposopathy and Cardiometabolic Diseases?
Abstract
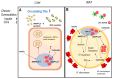
Angiopoietin-like proteins, namely ANGPTL3-4-8, are known as regulators of lipid metabolism. However, recent evidence points towards their involvement in the regulation of adipose tissue function. Alteration of adipose tissue functions (also called adiposopathy) is considered the main inducer of metabolic syndrome (MS) and its related complications. In this review, we intended to analyze available evidence derived from experimental and human investigations highlighting the contribution of ANGPTLs in the regulation of adipocyte metabolism, as well as their potential role in common cardiometabolic alterations associated with adiposopathy. We finally propose a model of ANGPTLs-based adipose tissue dysfunction, possibly linking abnormalities in the angiopoietins to the induction of adiposopathy and its related disorders.
Keywords: ANGPTL3; ANGPTL4; ANGPTL8; adipose tissue; adiposopathy; brown adipose tissue.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

