PhenQE8, a Novel Ligand of the Human Telomeric Quadruplex
- PMID: 33451070
- PMCID: PMC7828518
- DOI: 10.3390/ijms22020749
PhenQE8, a Novel Ligand of the Human Telomeric Quadruplex
Abstract
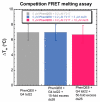
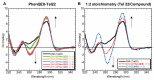
A novel quadruplex ligand based on 1,10-phenanthroline and incorporating two guanyl hydrazone functionalities, PhenQE8, is reported herein. Synthetic access was gained in a two-step procedure with an overall yield of 61%. X-ray diffraction studies revealed that PhenQE8 can adopt an extended conformation that may be optimal to favor recognition of quadruplex DNA. DNA interactions with polymorphic G-quadruplex telomeric structures were studied by different techniques, such as Fluorescence resonance energy transfer (FRET) DNA melting assays, circular dichroism and equilibrium dialysis. Our results reveal that the novel ligand PhenQE8 can efficiently recognize the hybrid quadruplex structures of the human telomeric DNA, with high binding affinity and quadruplex/duplex selectivity. Moreover, the compound shows significant cytotoxic activity against a selected panel of cultured tumor cells (PC-3, HeLa and MCF-7), whereas its cytotoxicity is considerably lower in healthy human cells (HFF-1 and RPWE-1).
Keywords: 1,10-phenanthroline; DNA interactions; antitumor agents; guanyl hydrazones; human telomeric quadruplex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Neidle S. Quadruplex nucleic acids as targets for anticancer therapeutics. Nat. Rev. Chem. 2017;1:41. doi: 10.1038/s41570-017-0041. - DOI
-
- Tian T., Chen Y.Q., Wang S.R., Zhou X. G-Quadruplex: A Regulator of Gene Expression and Its Chemical Targeting. Chem. 2018;4:1314–1344. doi: 10.1016/j.chempr.2018.02.014. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

