FOLFOX Therapy Induces Feedback Upregulation of CD44v6 through YB-1 to Maintain Stemness in Colon Initiating Cells
- PMID: 33451103
- PMCID: PMC7828641
- DOI: 10.3390/ijms22020753
FOLFOX Therapy Induces Feedback Upregulation of CD44v6 through YB-1 to Maintain Stemness in Colon Initiating Cells
Abstract
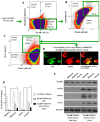
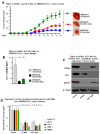
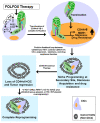
Cancer initiating cells (CICs) drive tumor formation and drug-resistance, but how they develop drug-resistance characteristics is not well understood. In this study, we demonstrate that chemotherapeutic agent FOLFOX, commonly used for drug-resistant/metastatic colorectal cancer (CRC) treatment, induces overexpression of CD44v6, MDR1, and oncogenic transcription/translation factor Y-box-binding protein-1 (YB-1). Our study revealed that CD44v6, a receptor for hyaluronan, increased the YB-1 expression through PGE2/EP1-mTOR pathway. Deleting CD44v6, and YB-1 by the CRISPR/Cas9 system attenuates the in vitro and in vivo tumor growth of CICs from FOLFOX resistant cells. The results of DNA:CD44v6 immunoprecipitated complexes by ChIP (chromatin-immunoprecipitation) assay showed that CD44v6 maintained the stemness traits by promoting several antiapoptotic and stemness genes, including cyclin-D1, BCL2, FZD1, GINS-1, and MMP9. Further, computer-based analysis of the clones obtained from the DNA:CD44v6 complex revealed the presence of various consensus binding sites for core stemness-associated transcription factors "CTOS" (c-Myc, TWIST1, OCT4, and SOX2). Simultaneous expressions of CD44v6 and CTOS in CD44v6 knockout CICs reverted differentiated CD44v6-knockout CICs into CICs. Finally, this study for the first time describes a positive feedback loop that couples YB-1 induction and CD44 alternative splicing to sustain the MDR1 and CD44v6 expressions, and CD44v6 is required for the reversion of differentiated tumor cells into CICs.
Keywords: CD44v6; CD44v6 CRISPR/Cas9 knockout; CD44v6-therapy; CIC; MDR1; YB-1; YB-1 CRISPR/Cas9 knockout; colorectal cancer (CRC); stemness genes.
Conflict of interest statement
The authors declare that they have no conflict of interest with the contents of this article.
Figures





Similar articles
-
Chemotherapy induces feedback up-regulation of CD44v6 in colorectal cancer initiating cells through β-catenin/MDR1 signaling to sustain chemoresistance.Front Oncol. 2022 Oct 18;12:906260. doi: 10.3389/fonc.2022.906260. eCollection 2022. Front Oncol. 2022. PMID: 36330477 Free PMC article.
-
Schlafen-3 decreases cancer stem cell marker expression and autocrine/juxtacrine signaling in FOLFOX-resistant colon cancer cells.Am J Physiol Gastrointest Liver Physiol. 2011 Aug;301(2):G347-55. doi: 10.1152/ajpgi.00403.2010. Epub 2011 May 19. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21596996 Free PMC article.
-
Cancer-initiating cells derived from human rectal adenocarcinoma tissues carry mesenchymal phenotypes and resist drug therapies.Cell Death Dis. 2013 Oct 3;4(10):e828. doi: 10.1038/cddis.2013.337. Cell Death Dis. 2013. PMID: 24091671 Free PMC article.
-
[Predictive marker for the efficacy of FOLFOX treatment in colon cancer (TS, ERCC1 etc)].Nihon Rinsho. 2011 Apr;69 Suppl 3:494-9. Nihon Rinsho. 2011. PMID: 22214010 Review. Japanese. No abstract available.
-
Activation of Matrix Hyaluronan-Mediated CD44 Signaling, Epigenetic Regulation and Chemoresistance in Head and Neck Cancer Stem Cells.Int J Mol Sci. 2017 Aug 24;18(9):1849. doi: 10.3390/ijms18091849. Int J Mol Sci. 2017. PMID: 28837080 Free PMC article. Review.
Cited by
-
Chemotherapy induces feedback up-regulation of CD44v6 in colorectal cancer initiating cells through β-catenin/MDR1 signaling to sustain chemoresistance.Front Oncol. 2022 Oct 18;12:906260. doi: 10.3389/fonc.2022.906260. eCollection 2022. Front Oncol. 2022. PMID: 36330477 Free PMC article.
-
Comparative analysis between 2D and 3D colorectal cancer culture models for insights into cellular morphological and transcriptomic variations.Sci Rep. 2023 Oct 26;13(1):18380. doi: 10.1038/s41598-023-45144-w. Sci Rep. 2023. PMID: 37884554 Free PMC article.
-
Application of CRISPR-Cas9 gene editing technology in basic research, diagnosis and treatment of colon cancer.Front Endocrinol (Lausanne). 2023 Mar 20;14:1148412. doi: 10.3389/fendo.2023.1148412. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37020597 Free PMC article. Review.
-
Drug Resistance in Medulloblastoma Is Driven by YB-1, ABCB1 and a Seven-Gene Drug Signature.Cancers (Basel). 2023 Feb 8;15(4):1086. doi: 10.3390/cancers15041086. Cancers (Basel). 2023. PMID: 36831428 Free PMC article.
-
Metabolic reprogramming and therapeutic resistance in primary and metastatic breast cancer.Mol Cancer. 2024 Nov 21;23(1):261. doi: 10.1186/s12943-024-02165-x. Mol Cancer. 2024. PMID: 39574178 Free PMC article. Review.
References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

