Impact of CNS Diseases on Drug Delivery to Brain Extracellular and Intracellular Target Sites in Human: A "WHAT-IF" Simulation Study
- PMID: 33451111
- PMCID: PMC7828633
- DOI: 10.3390/pharmaceutics13010095
Impact of CNS Diseases on Drug Delivery to Brain Extracellular and Intracellular Target Sites in Human: A "WHAT-IF" Simulation Study
Abstract
The blood-brain barrier (BBB) is equipped with unique physical and functional processes that control central nervous system (CNS) drug transport and the resulting concentration-time profiles (PK). In CNS diseases, the altered BBB and CNS pathophysiology may affect the CNS PK at the drug target sites in the brain extracellular fluid (brainECF) and intracellular fluid (brainICF) that may result in changes in CNS drug effects. Here, we used our human CNS physiologically-based PK model (LeiCNS-PK3.0) to investigate the impact of altered cerebral blood flow (CBF), tight junction paracellular pore radius (pararadius), brainECF volume, and pH of brainECF (pHECF) and of brainICF (pHICF) on brainECF and brainICF PK for 46 small drugs with distinct physicochemical properties. LeiCNS-PK3.0 simulations showed a drug-dependent effect of the pathophysiological changes on the rate and extent of BBB transport and on brainECF and brainICF PK. Altered pararadius, pHECF, and pHICF affected both the rate and extent of BBB drug transport, whereas changes in CBF and brainECF volume modestly affected the rate of BBB drug transport. While the focus is often on BBB paracellular and active transport processes, this study indicates that also changes in pH should be considered for their important implications on brainECF and brainICF target site PK.
Keywords: CNS diseases; blood–brain barrier; brain pharmacokinetics; passive transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
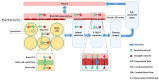

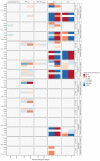
References
-
- Loryan I., Sinha V., Mackie C., Van Peer A., Drinkenburg W.H., Vermeulen A., Heald D., Hammarlund-Udenaes M., Wassvik C.M. Molecular properties determining unbound intracellular and extracellular brain exposure of CNS drug candidates. Mol. Pharm. 2015;12:520–532. doi: 10.1021/mp5005965. - DOI - PubMed
-
- Ketharanathan N., Yamamoto Y., Rohlwink U.K., Wildschut E.D., Mathôt R.A.A., De Lange E.C.M., De Wildt S.N., Argent A.C., Tibboel D., Figaji A.A. Combining Brain Microdialysis and Translational Pharmacokinetic Modeling to Predict Drug Concentrations in Pediatric Severe Traumatic Brain Injury: The Next Step Toward Evidence-Based Pharmacotherapy? J. Neurotrauma. 2019;36:111–117. doi: 10.1089/neu.2017.5588. - DOI - PubMed
-
- Bouw R., Ederoth P., Lundberg J., Ungerstedt U., Nordström C.-H., Hammarlund-Udenaes M. Increased blood–brain barrier permeability of morphine in a patient with severe brain lesions as determined by microdialysis. Acta Anaesthesiol. Scand. 2001;45:390–392. doi: 10.1034/j.1399-6576.2001.045003390.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

