The Landscape of AhR Regulators and Coregulators to Fine-Tune AhR Functions
- PMID: 33451129
- PMCID: PMC7828596
- DOI: 10.3390/ijms22020757
The Landscape of AhR Regulators and Coregulators to Fine-Tune AhR Functions
Abstract
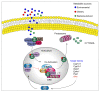
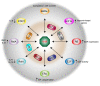
The aryl-hydrocarbon receptor (AhR) is a ligand-activated transcription factor that mediates numerous cellular responses. Originally investigated in toxicology because of its ability to bind environmental contaminants, AhR has attracted enormous attention in the field of immunology in the last 20 years. In addition, the discovery of endogenous and plant-derived ligands points to AhR also having a crucial role in normal cell physiology. Thus, AhR is emerging as a promiscuous receptor that can mediate either toxic or physiologic effects upon sensing multiple exogenous and endogenous molecules. Within this scenario, several factors appear to contribute to the outcome of gene transcriptional regulation by AhR, including the nature of the ligand as such and its further metabolism by AhR-induced enzymes, the local tissue microenvironment, and the presence of coregulators or specific transcription factors in the cell. Here, we review the current knowledge on the array of transcription factors and coregulators that, by interacting with AhR, tune its transcriptional activity in response to endogenous and exogenous ligands.
Keywords: arylhydrocarbon receptor (AhR); coregulators; immune regulation; nuclear coactivator; transcription factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

