A Closer Look at Estrogen Receptor Mutations in Breast Cancer and Their Implications for Estrogen and Antiestrogen Responses
- PMID: 33451133
- PMCID: PMC7828590
- DOI: 10.3390/ijms22020756
A Closer Look at Estrogen Receptor Mutations in Breast Cancer and Their Implications for Estrogen and Antiestrogen Responses
Abstract
Breast cancer (BC) is the most common cancer among women worldwide. More than 70% of BC cases express estrogen receptor alpha (ERα), a central transcription factor that stimulates the proliferation of breast cancer cells, usually in the presence of estrogen. While most cases of ER-positive BC initially respond to antiestrogen therapies, a high percentage of cases develop resistance to treatment over time. The recent discovery of mutated forms of ERα that result in constitutively active forms of the receptor in the metastatic-resistance stage of BC has provided a strong rationale for the development of new antiestrogens. These molecules targeting clinically relevant ERα mutants and a combination with other pharmacological inhibitors of specific pathways may constitute alternative treatments to improve clinical practice in the fight against metastatic-resistant ER-positive BC. In this review, we summarize the latest advances regarding the particular involvement of point mutations of ERα in endocrine resistance. We also discuss the involvement of synonymous ERα mutations with respect to co-translational folding of the receptor and ribosome biogenesis in breast carcinogenesis.
Keywords: breast cancer; endocrine resistance; estrogen receptor; mutation; receptor folding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
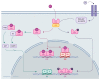
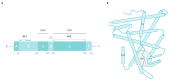
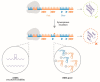
References
-
- Lakhani S., Ellis I., Schnitt S., Tan P., van de Vijver M. WHO Classification of Tumours of the Breast. 4th ed. International Agency for Research on Cancer; Lyon, France: 2012.
-
- Bertucci F., Finetti P., Rougemont J., Charafe-Jauffret E., Cervera N., Tarpin C., Nguyen C., Xerri L., Houlgatte R., Jacquemier J., et al. Gene Expression Profiling Identifies Molecular Subtypes of Inflammatory Breast Cancer. Cancer Res. 2005;65:2170–2178. doi: 10.1158/0008-5472.CAN-04-4115. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

