Antivirals blocking entry of enteroviruses and therapeutic potential
- PMID: 33451326
- PMCID: PMC7811253
- DOI: 10.1186/s12929-021-00708-8
Antivirals blocking entry of enteroviruses and therapeutic potential
Abstract
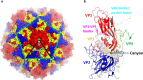
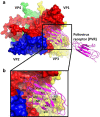
Viruses from the genus Enterovirus (EV) of the Picornaviridae family are known to cause diseases such as hand foot and mouth disease (HFMD), respiratory diseases, encephalitis and myocarditis. The capsid of EV is an attractive target for the development of direct-acting small molecules that can interfere with viral entry. Some of the capsid binders have been evaluated in clinical trials but the majority have failed due to insufficient efficacy or unacceptable off-target effects. Furthermore, most of the capsid binders exhibited a low barrier to resistance. Alternatively, host-targeting inhibitors such as peptides derived from the capsid of EV that can recognize cellular receptors have been identified. However, the majority of these peptides displayed low anti-EV potency (µM range) as compared to the potency of small molecule compounds (nM range). Nonetheless, the development of anti-EV peptides is warranted as they may complement the small-molecules in a drug combination strategy to treat EVs. Lastly, structure-based approach to design antiviral peptides should be utilized to unearth potent anti-EV peptides.
Keywords: Antiviral compound; Antiviral peptide; Enterovirus; Picornaviridae.
Conflict of interest statement
Not applicable.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

