Interfollicular epidermal stem-like cells for the recreation of the hair follicle epithelial compartment
- PMID: 33451331
- PMCID: PMC7811263
- DOI: 10.1186/s13287-020-02104-9
Interfollicular epidermal stem-like cells for the recreation of the hair follicle epithelial compartment
Erratum in
-
Correction: Interfollicular epidermal stem-like cells for the recreation of the hair follicle epithelial compartment.Stem Cell Res Ther. 2023 Oct 21;14(1):302. doi: 10.1186/s13287-023-03509-y. Stem Cell Res Ther. 2023. PMID: 37865805 Free PMC article. No abstract available.
Abstract
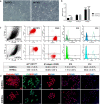
Background: Hair follicle (HF) development and growth are dependent on epithelial-mesenchymal interactions (EMIs). Dermal papilla (DP) cells are recognized as the key inductive mesenchymal player, but the ideal source of receptive keratinocytes for human HF regeneration is yet to be defined. We herein investigated whether human interfollicular epidermal keratinocytes with stem-like features (EpSlKCs), characterized by a α6bri/CD71dim expression, can replace human hair follicular keratinocytes (HHFKCs) for the recreation of the HF epithelium and respective EMIs.
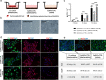
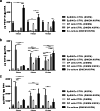
Methods: The α6bri/CD71dim cellular fraction was selected from the whole interfollicular keratinocyte population through fluorescence-activated cell sorting and directly compared with follicular keratinocytes in terms of their proliferative capacity and phenotype. The crosstalk with DP cells was studied in an indirect co-culture system, and EpSlKC hair forming capacity tested in a hair reconstitution assay when combined with DP cells.
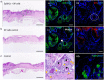
Results: EpSlKCs exhibited a phenotypic profile similar to follicular keratinocytes and were capable of increasing DP cell proliferation and, for short co-culture times, the number of alkaline phosphatase-active cells, suggesting an improvement of their inductivity. Moreover, the recreation of immature HFs and sebaceous glands was observed after EpSlKC and DP cell co-grafting in nude mice.
Conclusions: Our results suggest that EpSlKCs are akin to follicular keratinocytes and can crosstalk with DP cells, contributing to HF morphogenesis in vivo, thus representing an attractive epithelial cell source for hair regeneration strategies.
Keywords: Dermal papilla cells; Epidermal keratinocytes with stem-like features; Epithelial-mesenchymal interactions; Hair follicle; Sebaceous gland.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Rescuing key native traits in cultured dermal papilla cells for human hair regeneration.J Adv Res. 2020 Nov 4;30:103-112. doi: 10.1016/j.jare.2020.10.006. eCollection 2021 May. J Adv Res. 2020. PMID: 34026290 Free PMC article.
-
Stress-induced premature senescence of dermal papilla cells compromises hair follicle epithelial-mesenchymal interaction.J Dermatol Sci. 2017 May;86(2):114-122. doi: 10.1016/j.jdermsci.2017.01.003. Epub 2017 Jan 5. J Dermatol Sci. 2017. PMID: 28117106
-
Hair follicle regeneration using grafted rodent and human cells.J Invest Dermatol. 2007 Sep;127(9):2106-15. doi: 10.1038/sj.jid.5700823. Epub 2007 Apr 12. J Invest Dermatol. 2007. PMID: 17429436
-
Strategies to enhance epithelial-mesenchymal interactions for human hair follicle bioengineering.J Dermatol Sci. 2013 May;70(2):78-87. doi: 10.1016/j.jdermsci.2013.02.004. Epub 2013 Feb 28. J Dermatol Sci. 2013. PMID: 23557720 Review.
-
Hair Regeneration Methods Using Cells Derived from Human Hair Follicles and Challenges to Overcome.Cells. 2024 Dec 25;14(1):7. doi: 10.3390/cells14010007. Cells. 2024. PMID: 39791708 Free PMC article. Review.
Cited by
-
Epidermal-dermal coupled spheroids are important for tissue pattern regeneration in reconstituted skin explant cultures.NPJ Regen Med. 2023 Nov 23;8(1):65. doi: 10.1038/s41536-023-00340-0. NPJ Regen Med. 2023. PMID: 37996466 Free PMC article.
-
Molecular chaos under the skin: Epigenetic reprogramming in hidradenitis suppurativa.Proc Natl Acad Sci U S A. 2024 Jan 2;121(1):e2319797121. doi: 10.1073/pnas.2319797121. Epub 2023 Dec 26. Proc Natl Acad Sci U S A. 2024. PMID: 38147564 Free PMC article. No abstract available.
-
Recreation of a hair follicle regenerative microenvironment: Successes and pitfalls.Bioeng Transl Med. 2021 Jun 23;7(1):e10235. doi: 10.1002/btm2.10235. eCollection 2022 Jan. Bioeng Transl Med. 2021. PMID: 35079623 Free PMC article. Review.
-
Multilayered Gel-Spotting Device for In Vitro Reconstruction of Hair Follicle-like Microstructure.Micromachines (Basel). 2023 Aug 22;14(9):1651. doi: 10.3390/mi14091651. Micromachines (Basel). 2023. PMID: 37763814 Free PMC article.
-
Epigenetic switch reshapes epithelial progenitor cell signatures and drives inflammatory pathogenesis in hidradenitis suppurativa.Proc Natl Acad Sci U S A. 2023 Dec 5;120(49):e2315096120. doi: 10.1073/pnas.2315096120. Epub 2023 Nov 27. Proc Natl Acad Sci U S A. 2023. PMID: 38011564 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

