Missing clinical trial data: the evidence gap in primary data for potential COVID-19 drugs
- PMID: 33451350
- PMCID: PMC7809643
- DOI: 10.1186/s13063-021-05024-y
Missing clinical trial data: the evidence gap in primary data for potential COVID-19 drugs
Abstract
Background: Several drugs are being repurposed for the treatment of the coronavirus disease 2019 (COVID-19) pandemic based on in vitro or early clinical findings. As these drugs are being used in varied regimens and dosages, it is important to enable synthesis of existing safety data from clinical trials. However, availability of safety information is limited by a lack of timely reporting of overall clinical trial results on public registries or through academic publication. We aimed to analyse the evidence gap in this data by conducting a rapid review of results posting on ClinicalTrials.gov and in academic publications to quantify the number of trials missing results for drugs potentially being repurposed for COVID-19.
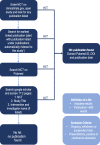
Methods: ClinicalTrials.gov was searched for 19 drugs that have been identified as potential treatments for COVID-19. Relevant clinical trials for any prior indication were listed by identifier (NCT number) and checked for results and for timely result reporting (within 395 days of the primary completion date). Additionally, PubMed and Google Scholar were searched to identify publications of results not listed on the registry. A second, blinded search of 10% of trials was conducted to assess reviewer concordance.
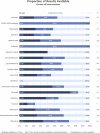
Results: Of 3754 completed trials, 1516 (40.4%) did not post results on ClinicalTrials.gov or in the academic literature. Tabular results were available on ClinicalTrials.gov for 1172 (31.2%) completed trials. A further 1066 (28.4%) had published results in the academic literature, but did not report results on ClinicalTrials.gov . Key drugs missing clinical trial results include hydroxychloroquine (37.0% completed trials unreported), favipiravir (77.8%) and lopinavir (40.5%).
Conclusions: There is an important evidence gap for the safety of drugs being repurposed for COVID-19. This uncertainty could cause unnecessary additional morbidity and mortality during the pandemic. We recommend caution in experimental drug use for non-severe disease and urge clinical trial sponsors to report missing results retrospectively.
Keywords: Adverse events; COVID-19 treatment; Clinical trial transparency; Coronavirus; Repurposed drugs; Safety information.
Conflict of interest statement
Three of the co-authors on this paper are part of Universities Allied for Essential Medicines U.K. However, views expressed in this paper are not necessarily that of Universities Allied for Essential Medicines Europe.
Figures

Similar articles
-
Characteristics of COVID-19 clinical trials registered with ClinicalTrials.gov: cross-sectional analysis.BMJ Open. 2020 Sep 17;10(9):e041276. doi: 10.1136/bmjopen-2020-041276. BMJ Open. 2020. PMID: 32948577 Free PMC article.
-
A Trial of Favipiravir and Hydroxychloroquine combination in Adults Hospitalized with moderate and severe Covid-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 31;21(1):904. doi: 10.1186/s13063-020-04825-x. Trials. 2020. PMID: 33129363 Free PMC article.
-
[The praise of uncertainty: a systematic living review to evaluate the efficacy and safety of drug treatments for patients with covid-19.].Recenti Prog Med. 2021 Mar;112(3):195-206. doi: 10.1701/3565.35458. Recenti Prog Med. 2021. PMID: 33687358 Italian.
-
Clinical Trials of Repurposed Antivirals for SARS-CoV-2.Antimicrob Agents Chemother. 2020 Aug 20;64(9):e01101-20. doi: 10.1128/AAC.01101-20. Print 2020 Aug 20. Antimicrob Agents Chemother. 2020. PMID: 32631826 Free PMC article. Review.
-
Effectiveness of Interferon Beta 1a, compared to Interferon Beta 1b and the usual therapeutic regimen to treat adults with moderate to severe COVID-19: structured summary of a study protocol for a randomized controlled trial.Trials. 2020 Jun 3;21(1):473. doi: 10.1186/s13063-020-04382-3. Trials. 2020. PMID: 32493468 Free PMC article.
Cited by
-
[Optimisation of risk and crisis communication of governments, authorities and public health institutions-challenges in long-lasting crises illustrated by the COVID-19 pandemic].Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2023 Aug;66(8):930-939. doi: 10.1007/s00103-023-03708-1. Epub 2023 Jun 6. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2023. PMID: 37280440 Free PMC article. Review. German.
-
Challenges and Lessons Learned From COVID-19 Trials: Should We Be Doing Clinical Trials Differently?Can J Cardiol. 2021 Sep;37(9):1353-1364. doi: 10.1016/j.cjca.2021.05.009. Epub 2021 May 30. Can J Cardiol. 2021. PMID: 34077789 Free PMC article. Review.
-
Characteristics of COVID-19 clinical trials registered with ClinicalTrials.gov: cross-sectional analysis.BMJ Open. 2020 Sep 17;10(9):e041276. doi: 10.1136/bmjopen-2020-041276. BMJ Open. 2020. PMID: 32948577 Free PMC article.
-
Clinical trial reporting performance of thirty UK universities on ClinicalTrials.gov-evaluation of a new tracking tool for the US clinical trial registry.Trials. 2021 Jun 1;22(1):375. doi: 10.1186/s13063-021-05330-5. Trials. 2021. PMID: 34074329 Free PMC article.
-
The Drug Repurposing for COVID-19 Clinical Trials Provide Very Effective Therapeutic Combinations: Lessons Learned From Major Clinical Studies.Front Pharmacol. 2021 Nov 18;12:704205. doi: 10.3389/fphar.2021.704205. eCollection 2021. Front Pharmacol. 2021. PMID: 34867318 Free PMC article. Review.
References
-
- COVID-19 Map - Johns Hopkins Coronavirus Resource Center.
-
- Kalil AC. Treating COVID-19 - off-label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA. 2020;323(19):1897–8. 10.1001/jama.2020.4742. - PubMed
-
- Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for Coronavirus Disease 2019 (COVID-19): a review. JAMA. 2020;323(18):1824–36. 10.1001/jama.2020.6019. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

