Longitudinal amyloid and tau accumulation in autosomal dominant Alzheimer's disease: findings from the Colombia-Boston (COLBOS) biomarker study
- PMID: 33451357
- PMCID: PMC7811244
- DOI: 10.1186/s13195-020-00765-5
Longitudinal amyloid and tau accumulation in autosomal dominant Alzheimer's disease: findings from the Colombia-Boston (COLBOS) biomarker study
Abstract
Background: Neuroimaging studies of autosomal dominant Alzheimer's disease (ADAD) enable characterization of the trajectories of cerebral amyloid-β (Aβ) and tau accumulation in the decades prior to clinical symptom onset. Longitudinal rates of regional tau accumulation measured with positron emission tomography (PET) and their relationship with other biomarker and cognitive changes remain to be fully characterized in ADAD.
Methods: Fourteen ADAD mutation carriers (Presenilin-1 E280A) and 15 age-matched non-carriers from the Colombian kindred underwent 2-3 sessions of Aβ (11C-Pittsburgh compound B) and tau (18F-flortaucipir) PET, structural magnetic resonance imaging, and neuropsychological evaluation over a 2-4-year follow-up period. Annualized rates of change for imaging and cognitive variables were compared between carriers and non-carriers, and relationships among baseline measurements and rates of change were assessed within carriers.
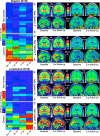
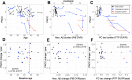
Results: Longitudinal measurements were consistent with a sequence of ADAD-related changes beginning with Aβ accumulation (16 years prior to expected symptom onset, EYO), followed by entorhinal cortex (EC) tau (9 EYO), neocortical tau (6 EYO), hippocampal atrophy (6 EYO), and cognitive decline (4 EYO). Rates of tau accumulation among carriers were most rapid in parietal neocortex (~ 9%/year). EC tau PET signal at baseline was a significant predictor of subsequent neocortical tau accumulation and cognitive decline within carriers.
Conclusions: Our results are consistent with the sequence of biological changes in ADAD implied by cross-sectional studies and highlight the importance of EC tau as an early biomarker and a potential link between Aβ burden and neocortical tau accumulation in ADAD.
Keywords: Alzheimer’s; Amyloid; Autosomal-Dominant; Imaging; Longitudinal; Tau.
Conflict of interest statement
Dr. Reiman reports receiving personal fees as a Scientific Advisor to Roche Diagnostics (travel expenses only), MagQ, Avid Radiopharmaceuticals, and is a share-holding co-founder of ALZPath, outside the submitted work. In addition, he is the inventor of a patent issued to Banner Health, which involves the use of biomarker endpoints in at-risk persons to accelerate the evaluation of Alzheimer’s disease prevention therapies and is outside the submitted work. Drs. Reiman and Lopera are principal investigators of the Alzheimer’s Prevention Initiative (API) Autosomal Dominant AD Trial, which is supported by NIA, philanthropy, Genentech, and Roche. Dr. Sperling is a site principal investigator or coinvestigator for Avid, Bristol-Myers Squibb, Pfizer, and Janssen Alzheimer Immunotherapy clinical trials. She receives travel funding and honoraria from AC Immune, Janssen, and Roche. She consults for Biogen, Roche, AC Immune, Eisai, Takeda, Neurocentria, and Janssen. Spouse consults for Novartis, AC Immune, and Janssen. Dr. Johnson has provided consulting services for Novartis, Biogen, and Eli Lilly. All other co-authors have no competing interests or disclosures relevant to the manuscript.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG031581/AG/NIA NIH HHS/United States
- R01 AG054671/AG/NIA NIH HHS/United States
- R01 AG027435/AG/NIA NIH HHS/United States
- DP5 OD019833/OD/NIH HHS/United States
- R01 AG046396/AG/NIA NIH HHS/United States
- R01 AG062559/AG/NIA NIH HHS/United States
- K23 AG058805/AG/NIA NIH HHS/United States
- P30 AG062421/AG/NIA NIH HHS/United States
- P01 AG036694/AG/NIA NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- U19 AG010483/AG/NIA NIH HHS/United States
- F31 AG062158/AG/NIA NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

