The Golgin Protein RUD3 Regulates Fusarium graminearum Growth and Virulence
- PMID: 33452023
- PMCID: PMC8104996
- DOI: 10.1128/AEM.02522-20
The Golgin Protein RUD3 Regulates Fusarium graminearum Growth and Virulence
Abstract
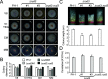
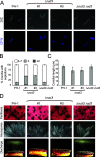
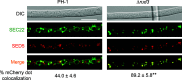
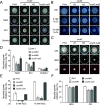
Golgins are coiled-coil proteins that play prominent roles in maintaining the structure and function of the Golgi complex. However, the role of golgin proteins in phytopathogenic fungi remains poorly understood. In this study, we functionally characterized the Fusarium graminearum golgin protein RUD3, a homolog of ScRUD3/GMAP-210 in Saccharomyces cerevisiae and mammalian cells. Cellular localization observation revealed that RUD3 is located in the cis-Golgi. Deletion of RUD3 caused defects in vegetative growth, ascospore discharge, deoxynivalenol (DON) production, and virulence. Moreover, the Δrud3 mutant showed reduced expression of tri genes and impairment of the formation of toxisomes, both of which play essential roles in DON biosynthesis. We further used green fluorescent protein (GFP)-tagged SNARE protein SEC22 (SEC22-GFP) as a tool to study the transport between the endoplasmic reticulum (ER) and Golgi and observed that SEC22-GFP was retained in the cis-Golgi in the Δrud3 mutant. RUD3 contains the coiled coil (CC), GRAB-associated 2 (GA2), GRIP-related Arf binding (GRAB), and GRAB-associated 1 (GA1) domains, which except for GA1, are indispensable for normal localization and function of RUD3, whereas only CC is essential for normal RUD3-RUD3 interaction. Together, these results demonstrate how the golgin protein RUD3 mediates retrograde trafficking in the ER-to-Golgi pathway and is necessary for growth, ascospore discharge, DON biosynthesis, and pathogenicity in F. graminearumIMPORTANCEFusarium head blight (FHB) caused by the fungal pathogen Fusarium graminearum is an economically important disease of wheat and other small grain cereal crops worldwide, and limited effective control strategies are available. A better understanding of the regulation mechanisms of F. graminearum development, deoxynivalenol (DON) biosynthesis, and pathogenicity is therefore important for the development of effective control management of this disease. Golgins are attached via their extreme carboxy terminus to the Golgi membrane and are involved in vesicle trafficking and organelle maintenance in eukaryotic cells. In this study, we systematically characterized a highly conserved Golgin protein, RUD3, and found that it is required for vegetative growth, ascospore discharge, DON production, and pathogenicity in F. graminearum Our findings provide a comprehensive characterization of the golgin family protein RUD3 in plant-pathogenic fungus, which could help to identify a new potential target for effective control of this devastating disease.
Keywords: FgRud3; Fusarium graminearum; RUD3; cis-Golgi; golgin; intracellular protein trafficking; virulence.
Copyright © 2021 American Society for Microbiology.
Figures

Similar articles
-
The Dynamin-Like GTPase FgSey1 Plays a Critical Role in Fungal Development and Virulence in Fusarium graminearum.Appl Environ Microbiol. 2020 May 19;86(11):e02720-19. doi: 10.1128/AEM.02720-19. Print 2020 May 19. Appl Environ Microbiol. 2020. PMID: 32220839 Free PMC article.
-
Genome-Wide Characterization of PX Domain-Containing Proteins Involved in Membrane Trafficking-Dependent Growth and Pathogenicity of Fusarium graminearum.mBio. 2021 Dec 21;12(6):e0232421. doi: 10.1128/mBio.02324-21. Epub 2021 Dec 21. mBio. 2021. PMID: 34933449 Free PMC article.
-
The t-SNARE protein FgPep12, associated with FgVam7, is essential for ascospore discharge and plant infection by trafficking Ca2+ ATPase FgNeo1 between Golgi and endosome/vacuole in Fusarium graminearum.PLoS Pathog. 2019 May 8;15(5):e1007754. doi: 10.1371/journal.ppat.1007754. eCollection 2019 May. PLoS Pathog. 2019. PMID: 31067272 Free PMC article.
-
Advances in Understanding Fusarium graminearum: Genes Involved in the Regulation of Sexual Development, Pathogenesis, and Deoxynivalenol Biosynthesis.Genes (Basel). 2024 Apr 9;15(4):475. doi: 10.3390/genes15040475. Genes (Basel). 2024. PMID: 38674409 Free PMC article. Review.
-
Fusarium graminearum in Wheat-Management Strategies in Central Europe.Pathogens. 2025 Mar 8;14(3):265. doi: 10.3390/pathogens14030265. Pathogens. 2025. PMID: 40137750 Free PMC article. Review.
Cited by
-
The Endoplasmic Reticulum Membrane Protein Complex Is Important for Deoxynivalenol Production and the Virulence of Fusarium graminearum.J Fungi (Basel). 2025 Jan 31;11(2):108. doi: 10.3390/jof11020108. J Fungi (Basel). 2025. PMID: 39997402 Free PMC article.
-
Rhizobium Soaking Promoted Maize Growth by Altering Rhizosphere Microbiomes and Associated Functional Genes.Microorganisms. 2023 Jun 25;11(7):1654. doi: 10.3390/microorganisms11071654. Microorganisms. 2023. PMID: 37512827 Free PMC article.
-
Hypothetical protein FoDbp40 influences the growth and virulence of Fusarium oxysporum by regulating the expression of isocitrate lyase.Front Microbiol. 2022 Nov 28;13:1050637. doi: 10.3389/fmicb.2022.1050637. eCollection 2022. Front Microbiol. 2022. PMID: 36519161 Free PMC article.
-
The Endoplasmic Reticulum Cargo Receptor FgErv14 Regulates DON Production, Growth and Virulence in Fusarium graminearum.Life (Basel). 2022 May 27;12(6):799. doi: 10.3390/life12060799. Life (Basel). 2022. PMID: 35743830 Free PMC article.
-
UDP-Galactopyranose Mutase Mediates Cell Wall Integrity, Polarity Growth, and Virulence in Fusarium graminearum.Appl Environ Microbiol. 2023 Feb 28;89(2):e0123522. doi: 10.1128/aem.01235-22. Epub 2023 Jan 19. Appl Environ Microbiol. 2023. PMID: 36656025 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

