Modelling the cascade of biomarker changes in GRN-related frontotemporal dementia
- PMID: 33452053
- PMCID: PMC8053353
- DOI: 10.1136/jnnp-2020-323541
Modelling the cascade of biomarker changes in GRN-related frontotemporal dementia
Abstract
Objective: Progranulin-related frontotemporal dementia (FTD-GRN) is a fast progressive disease. Modelling the cascade of multimodal biomarker changes aids in understanding the aetiology of this disease and enables monitoring of individual mutation carriers. In this cross-sectional study, we estimated the temporal cascade of biomarker changes for FTD-GRN, in a data-driven way.
Methods: We included 56 presymptomatic and 35 symptomatic GRN mutation carriers, and 35 healthy non-carriers. Selected biomarkers were neurofilament light chain (NfL), grey matter volume, white matter microstructure and cognitive domains. We used discriminative event-based modelling to infer the cascade of biomarker changes in FTD-GRN and estimated individual disease severity through cross-validation. We derived the biomarker cascades in non-fluent variant primary progressive aphasia (nfvPPA) and behavioural variant FTD (bvFTD) to understand the differences between these phenotypes.
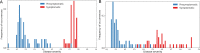
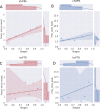
Results: Language functioning and NfL were the earliest abnormal biomarkers in FTD-GRN. White matter tracts were affected before grey matter volume, and the left hemisphere degenerated before the right. Based on individual disease severities, presymptomatic carriers could be delineated from symptomatic carriers with a sensitivity of 100% and specificity of 96.1%. The estimated disease severity strongly correlated with functional severity in nfvPPA, but not in bvFTD. In addition, the biomarker cascade in bvFTD showed more uncertainty than nfvPPA.
Conclusion: Degeneration of axons and language deficits are indicated to be the earliest biomarkers in FTD-GRN, with bvFTD being more heterogeneous in disease progression than nfvPPA. Our data-driven model could help identify presymptomatic GRN mutation carriers at risk of conversion to the clinical stage.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: RS-V received personal fees for participating in advisory meetings from Wave pharmaceuticals and Ionis.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
