The harsh microenvironment in early breast cancer selects for a Warburg phenotype
- PMID: 33452133
- PMCID: PMC7826394
- DOI: 10.1073/pnas.2011342118
The harsh microenvironment in early breast cancer selects for a Warburg phenotype
Abstract
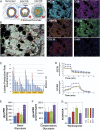
The harsh microenvironment of ductal carcinoma in situ (DCIS) exerts strong evolutionary selection pressures on cancer cells. We hypothesize that the poor metabolic conditions near the ductal center foment the emergence of a Warburg Effect (WE) phenotype, wherein cells rapidly ferment glucose to lactic acid, even in normoxia. To test this hypothesis, we subjected low-glycolytic breast cancer cells to different microenvironmental selection pressures using combinations of hypoxia, acidosis, low glucose, and starvation for many months and isolated single clones for metabolic and transcriptomic profiling. The two harshest conditions selected for constitutively expressed WE phenotypes. RNA sequencing analysis of WE clones identified the transcription factor KLF4 as potential inducer of the WE phenotype. In stained DCIS samples, KLF4 expression was enriched in the area with the harshest microenvironmental conditions. We simulated in vivo DCIS phenotypic evolution using a mathematical model calibrated from the in vitro results. The WE phenotype emerged in the poor metabolic conditions near the necrotic core. We propose that harsh microenvironments within DCIS select for a WE phenotype through constitutive transcriptional reprogramming, thus conferring a survival advantage and facilitating further growth and invasion.
Keywords: DCIS; Warburg Effect; adaptation; clonal selection; tumor evolution.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Gatenby R. A., Gillies R. J., Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 4, 891–899 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

