CXCL13 shapes immunoactive tumor microenvironment and enhances the efficacy of PD-1 checkpoint blockade in high-grade serous ovarian cancer
- PMID: 33452206
- PMCID: PMC7813306
- DOI: 10.1136/jitc-2020-001136
CXCL13 shapes immunoactive tumor microenvironment and enhances the efficacy of PD-1 checkpoint blockade in high-grade serous ovarian cancer
Abstract
Background: Most patients with high-grade serous ovarian cancer (HGSC) lack an effective response to immune checkpoint blockade, highlighting the need for more knowledge about what is required for successful treatment. As follicular cytotoxic CXCR5+CD8+ T cells are maintained by reinvigoration by immune checkpoint blockade in tumors, we attempted to reveal the relationship between CXCR5+CD8+ T cells and the tumor microenvironment to predict immunotherapy responses in HGSC.
Methods: 264 patients with HGSC from two cohorts and 340 HGSC cases from The Cancer Genome Atlas cohort were enrolled. Ex vivo and in vivo studies were conducted with human HGSC tumors and murine tumor models. The spatial correlation between CXC-chemokine ligand 13 (CXCL13), CXCR5, CD8, and CD20 was evaluated by immunohistochemistry and immunofluorescence. Survival was compared between different subsets of patients using Kaplan-Meier analysis. The therapeutic effect of CXCL13 and programmed cell death-1 (PD-1) blockade was validated using human HGSC tumors and murine models.
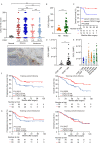
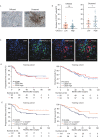
Results: High CXCL13 expression was associated with prolonged survival. Tumors with high CXCL13 expression exhibited increased infiltration of activated and CXCR5-expressing CD8+ T cells. Incubation with CXCL13 facilitated expansion and activation of CXCR5+CD8+ T cells ex vivo. CXCR5+CD8+ T cells appeared in closer proximity to CXCL13 in tumors and chemotaxis towards CXCL13 in vitro. The combination of CXCL13, CXCR5, and CD8+ T cells was an independent predictor for survival. In addition, CXCL13 was associated with clusters of CD20+ B cells. CD20+ B cells predicted better patient survival in the presence of CXCL13. Histological evaluation highlighted colocalization of CXCL13 with tertiary lymphoid structures (TLSs). TLSs carried prognostic benefit only in the presence of CXCL13. CXCL13 in combination with anti-PD-1 therapy retarded tumor growth in a CD8+ T-cell-dependent manner, resulting in increased infiltration of cytotoxic CD8+ T cells and CXCR5+CD8+ T cells.
Conclusions: These data define a critical role of CXCL13 in shaping antitumor microenvironment by facilitating the maintenance of CXCR5+CD8+ T cells in TLSs and support a clinical investigation for a combination of CXCL13 and PD-1 blockade therapy in HGSC.
Keywords: biomarkers; cytokines; tumor; tumor microenvironment.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures




Similar articles
-
High expression of CXCL13 predicts a favorable response to immunotherapy by upregulating CXCR5+CD8+ T-cell infiltration in gastric cancer.Front Immunol. 2025 May 8;16:1551259. doi: 10.3389/fimmu.2025.1551259. eCollection 2025. Front Immunol. 2025. PMID: 40406143 Free PMC article.
-
Enhanced Efficacy of Simultaneous PD-1 and PD-L1 Immune Checkpoint Blockade in High-Grade Serous Ovarian Cancer.Cancer Res. 2021 Jan 1;81(1):158-173. doi: 10.1158/0008-5472.CAN-20-1674. Epub 2020 Nov 6. Cancer Res. 2021. PMID: 33158814 Free PMC article.
-
Adaptive antitumor immune response stimulated by bio-nanoparticle based vaccine and checkpoint blockade.J Exp Clin Cancer Res. 2022 Apr 8;41(1):132. doi: 10.1186/s13046-022-02307-3. J Exp Clin Cancer Res. 2022. PMID: 35392977 Free PMC article.
-
Revisiting the CXCL13/CXCR5 axis in the tumor microenvironment in the era of single-cell omics: Implications for immunotherapy.Cancer Lett. 2024 Nov 28;605:217278. doi: 10.1016/j.canlet.2024.217278. Epub 2024 Sep 26. Cancer Lett. 2024. PMID: 39332588 Review.
-
CXCL13/CXCR5 signaling axis in cancer.Life Sci. 2019 Jun 15;227:175-186. doi: 10.1016/j.lfs.2019.04.053. Epub 2019 Apr 23. Life Sci. 2019. PMID: 31026453 Review.
Cited by
-
Lymphocyte activation gene 3 served as a potential prognostic and immunological biomarker across various cancer types: a clinical and pan-cancer analysis.Clin Transl Immunology. 2024 Oct 4;13(10):e70009. doi: 10.1002/cti2.70009. eCollection 2024. Clin Transl Immunology. 2024. PMID: 39372371 Free PMC article.
-
BCAT2 Shapes a Noninflamed Tumor Microenvironment and Induces Resistance to Anti-PD-1/PD-L1 Immunotherapy by Negatively Regulating Proinflammatory Chemokines and Anticancer Immunity.Adv Sci (Weinh). 2023 Mar;10(8):e2207155. doi: 10.1002/advs.202207155. Epub 2023 Jan 15. Adv Sci (Weinh). 2023. PMID: 36642843 Free PMC article.
-
Identification of CD8+ T Cell Related Biomarkers in Ovarian Cancer.Front Genet. 2022 May 27;13:860161. doi: 10.3389/fgene.2022.860161. eCollection 2022. Front Genet. 2022. PMID: 35711935 Free PMC article.
-
Targeted Mass Spectrometry Enables Multiplexed Quantification of Immunomodulatory Proteins in Clinical Biospecimens.Front Immunol. 2021 Nov 11;12:765898. doi: 10.3389/fimmu.2021.765898. eCollection 2021. Front Immunol. 2021. PMID: 34858420 Free PMC article.
-
Epithelial ovarian cancer is infiltrated by activated effector T cells co-expressing CD39, PD-1, TIM-3, CD137 and interacting with cancer cells and myeloid cells.Front Immunol. 2023 Oct 4;14:1212444. doi: 10.3389/fimmu.2023.1212444. eCollection 2023. Front Immunol. 2023. PMID: 37868997 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
