Identification of neuroblastoma cell lines with uncommon TAZ+/mesenchymal stromal cell phenotype with strong suppressive activity on natural killer cells
- PMID: 33452207
- PMCID: PMC7813384
- DOI: 10.1136/jitc-2020-001313
Identification of neuroblastoma cell lines with uncommon TAZ+/mesenchymal stromal cell phenotype with strong suppressive activity on natural killer cells
Abstract
Background: Neuroblastoma (NB) is the most common, extracranial childhood solid tumor arising from neural crest progenitor cells and is a primary cause of death in pediatric patients. In solid tumors, stromal elements recruited or generated by the cancer cells favor the development of an immune-suppressive microenvironment. Herein, we investigated in NB cell lines and in NB biopsies, the presence of cancer cells with mesenchymal phenotype and determined the immune-suppressive properties of these tumor cells on natural killer (NK) cells.
Methods: We assessed the mesenchymal stromal cell (MSC)-like phenotype and function of five human NB cell lines and the presence of this particular subset of neuroblasts in NB biopsies using flow-cytometry, immunohistochemistry, RT-qPCR, cytotoxicity assays, western blot and silencing strategy. We corroborated our data consulting a public gene-expression dataset.
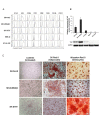
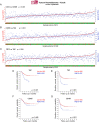
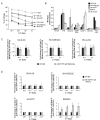
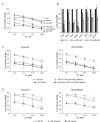
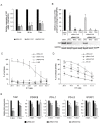
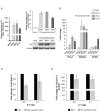
Results: Two NB cell lines, SK-N-AS and SK-N-BE(2)C, exhibited an unprecedented MSC phenotype (CD105+/CD90+/CD73+/CD29+/CD146+/GD2+/TAZ+). In these NB-MSCs, the ectoenzyme CD73 and the oncogenic/immune-regulatory transcriptional coactivator TAZ were peculiar markers. Their MSC-like nature was confirmed by their adipogenic and osteogenic differentiation potential. Immunohistochemical analysis confirmed the presence of neuroblasts with MSC phenotype (CD105+/CD73+/TAZ+). Moreover, a public gene-expression dataset revealed that, in stage IV NB, a higher expression of TAZ and CD105 strongly correlated with a poorer outcome.Among the NB-cell lines analyzed, only NB-MSCs exhibited multifactorial resistance to NK-mediated lysis, inhibition of activating NK receptors, signal adaptors and of NK-cell cytotoxicity through cell-cell contact mediated mechanisms. The latter property was controlled partially by TAZ, since its silencing in NB cells efficiently rescued NK-cell cytotoxic activity, while its overexpression induced opposite effects in non-NB-MSC cells.
Conclusions: We identified a novel NB immunoregulatory subset that: (i) displayed phenotypic and functional properties of MSC, (ii) mediated multifactorial resistance to NK-cell-induced killing and (iii) efficiently inhibited, in coculture, the cytotoxic activity of NK cells against target cells through a TAZ-dependent mechanism. These findings indicate that targeting novel cellular and molecular components may disrupt the immunomodulatory milieu of the NB microenvironment ameliorating the response to conventional treatments as well as to advanced immunotherapeutic approaches, including adoptive transfer of NK cells and chimeric antigen receptor T or NK cells.
Keywords: biomarkers; immune evation; killer cells; natural; neuroblastoma; tumor; tumor escape.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
