Transneuronal delivery of hyper-interleukin-6 enables functional recovery after severe spinal cord injury in mice
- PMID: 33452250
- PMCID: PMC7810685
- DOI: 10.1038/s41467-020-20112-4
Transneuronal delivery of hyper-interleukin-6 enables functional recovery after severe spinal cord injury in mice
Abstract
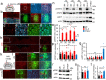
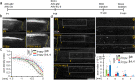
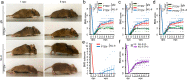
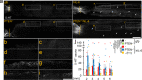
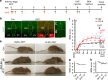
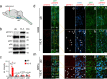
Spinal cord injury (SCI) often causes severe and permanent disabilities due to the regenerative failure of severed axons. Here we report significant locomotor recovery of both hindlimbs after a complete spinal cord crush. This is achieved by the unilateral transduction of cortical motoneurons with an AAV expressing hyper-IL-6 (hIL-6), a potent designer cytokine stimulating JAK/STAT3 signaling and axon regeneration. We find collaterals of these AAV-transduced motoneurons projecting to serotonergic neurons in both sides of the raphe nuclei. Hence, the transduction of cortical neurons facilitates the axonal transport and release of hIL-6 at innervated neurons in the brain stem. Therefore, this transneuronal delivery of hIL-6 promotes the regeneration of corticospinal and raphespinal fibers after injury, with the latter being essential for hIL-6-induced functional recovery. Thus, transneuronal delivery enables regenerative stimulation of neurons in the deep brain stem that are otherwise challenging to access, yet highly relevant for functional recovery after SCI.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

