The possible occurrence of iron-dependent anaerobic methane oxidation in an Archean Ocean analogue
- PMID: 33452366
- PMCID: PMC7810693
- DOI: 10.1038/s41598-021-81210-x
The possible occurrence of iron-dependent anaerobic methane oxidation in an Archean Ocean analogue
Abstract
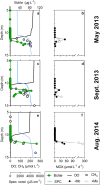
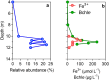
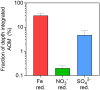
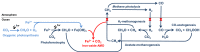
In the ferruginous and anoxic early Earth oceans, photoferrotrophy drove most of the biological production before the advent of oxygenic photosynthesis, but its association with ferric iron (Fe3+) dependent anaerobic methane (CH4) oxidation (AOM) has been poorly investigated. We studied AOM in Kabuno Bay, a modern analogue to the Archean Ocean (anoxic bottom waters and dissolved Fe concentrations > 600 µmol L-1). Aerobic and anaerobic CH4 oxidation rates up to 0.12 ± 0.03 and 51 ± 1 µmol L-1 d-1, respectively, were put in evidence. In the Fe oxidation-reduction zone, we observed high concentration of Bacteriochlorophyll e (biomarker of the anoxygenic photoautotrophs), which co-occurred with the maximum CH4 oxidation peaks, and a high abundance of Candidatus Methanoperedens, which can couple AOM to Fe3+ reduction. In addition, comparison of measured CH4 oxidation rates with electron acceptor fluxes suggest that AOM could mainly rely on Fe3+ produced by photoferrotrophs. Further experiments specifically targeted to investigate the interactions between photoferrotrophs and AOM would be of considerable interest. Indeed, ferric Fe3+-driven AOM has been poorly envisaged as a possible metabolic process in the Archean ocean, but this can potentially change the conceptualization and modelling of metabolic and geochemical processes controlling climate conditions in the Early Earth.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Borges AV, et al. Globally significant greenhouse-gas emissions from African inland waters. Nat. Geosci. 2015;8:637–642. doi: 10.1038/ngeo2486. - DOI
-
- Iversen N, Jørgensen B. Anaerobic methane oxidation rates at the sulfate-methane transition in marine sediments from Kattegat and Skagerrak (Denmark) Limnol. Oceanogr. 1985;30:944–955. doi: 10.4319/lo.1985.30.5.0944. - DOI
-
- Jørgensen BB, Weber A, Zopfi J. Sulfate reduction and anaerobic methane oxidation in Black Sea sediments. Deep-Sea Res. Pt. 2001;I(48):2097–2120. doi: 10.1016/S0967-0637(01)00007-3. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

