Clustered rapid induction of apoptosis limits ZIKV and DENV-2 proliferation in the midguts of Aedes aegypti
- PMID: 33452408
- PMCID: PMC7810730
- DOI: 10.1038/s42003-020-01614-9
Clustered rapid induction of apoptosis limits ZIKV and DENV-2 proliferation in the midguts of Aedes aegypti
Abstract
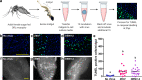
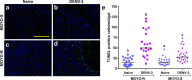
Inter-host transmission of pathogenic arboviruses such as dengue virus (DENV) and Zika virus (ZIKV) requires systemic infection of the mosquito vector. Successful systemic infection requires initial viral entry and proliferation in the midgut cells of the mosquito followed by dissemination to secondary tissues and eventual entry into salivary glands1. Lack of arbovirus proliferation in midgut cells has been observed in several Aedes aegypti strains2, but the midgut antiviral responses underlying this phenomenon are not yet fully understood. We report here that there is a rapid induction of apoptosis (RIA) in the Aedes aegypti midgut epithelium within 2 hours of infection with DENV-2 or ZIKV in both in vivo blood-feeding and ex vivo midgut infection models. Inhibition of RIA led to increased virus proliferation in the midgut, implicating RIA as an innate immune mechanism mediating midgut infection in this mosquito vector.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Caicedo PA, et al. Selection of Aedes aegypti (Diptera: Culicidae) strains that are susceptible or refractory to Dengue-2 virus. Can. Entomol. 2013;145:273–282. doi: 10.4039/tce.2012.105. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

