Systematic review of phase-I/II trials enrolling refractory and recurrent Ewing sarcoma: Actual knowledge and future directions to optimize the research
- PMID: 33452711
- PMCID: PMC7940237
- DOI: 10.1002/cam4.3712
Systematic review of phase-I/II trials enrolling refractory and recurrent Ewing sarcoma: Actual knowledge and future directions to optimize the research
Abstract
Background: Optimal Phase-II design to evaluate new therapies in refractory/relapsed Ewing sarcomas (ES) remains imperfectly defined.
Objectives: Recurrent/refractory ES phase-I/II trials analysis to improve trials design.
Methods: Comprehensive review of therapeutic trials registered on five databases (who.int/trialsearch, clinicaltrials.gov, clinicaltrialsregister.eu, e-cancer.fr, and umin.ac.jp) and/or published in PubMed/ASCO/ESMO websites, between 2005 and 2018, using the criterion: (Ewing sarcoma OR bone sarcoma OR sarcoma) AND (Phase-I or Phase-II).
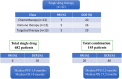
Results: The 146 trials identified (77 phase-I/II, 67 phase-II, and 2 phase-II/III) tested targeted (34%), chemo- (23%), immune therapies (19%), or combined therapies (24%). Twenty-three trials were ES specific and 48 had a specific ES stratum. Usually multicentric (88%), few trials were international (30%). Inclusion criteria cover the recurrent ES age range for only 12% of trials and allowed only accrual of measurable diseases (RECIST criteria). Single-arm design was the most frequent (88%) testing mainly single drugs (61%), only 5% were randomized. Primary efficacy outcome was response rate (RR=CR+PR; Complete+Partial response) (n = 116/146; 79%), rarely progression-free or overall survival (16% PFS and 3% OS). H0 and H1 hypotheses were variable (3%-25% and 20%-50%, respectively). The 62 published trials enrolled 827 ES patients. RR was poor (10%; 15 CR=1.7%, 68 PR=8.3%). Stable disease was the best response for 186 patients (25%). Median PFS/OS was of 1.9 (range 1.3-14.7) and 7.6 months (5-30), respectively. Eleven (18%) published trials were considered positive, with median RR/PFS/OS of 15% (7%-30%), 4.5 (1.3-10), and 16.6 months (6.9-30), respectively.
Conclusion: This review supports the need to develop the international randomized phase-II trials across all age ranges with PFS as primary endpoint.
Keywords: Ewing sarcoma; new cancer therapies; phase-I/II trials; trial design.
© 2021 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures
Similar articles
-
Historical time to disease progression and progression-free survival in patients with recurrent/refractory neuroblastoma treated in the modern era on Children's Oncology Group early-phase trials.Cancer. 2017 Dec 15;123(24):4914-4923. doi: 10.1002/cncr.30934. Epub 2017 Sep 8. Cancer. 2017. PMID: 28885700 Free PMC article. Review.
-
Which Factors Are Associated with Local Control and Survival of Patients with Localized Pelvic Ewing's Sarcoma? A Retrospective Analysis of Data from the Euro-EWING99 Trial.Clin Orthop Relat Res. 2020 Feb;478(2):290-302. doi: 10.1097/CORR.0000000000000962. Clin Orthop Relat Res. 2020. PMID: 31580267 Free PMC article.
-
Predictors of response, progression-free survival, and overall survival using NANT Response Criteria (v1.0) in relapsed and refractory high-risk neuroblastoma.Pediatr Blood Cancer. 2018 May;65(5):e26940. doi: 10.1002/pbc.26940. Epub 2018 Jan 19. Pediatr Blood Cancer. 2018. PMID: 29350464 Free PMC article.
-
Consensus recommendations for systemic therapies in the management of relapsed Ewing sarcoma: A report from the National Ewing Sarcoma Tumor Board.Cancer. 2024 Dec 1;130(23):4028-4039. doi: 10.1002/cncr.35537. Epub 2024 Aug 25. Cancer. 2024. PMID: 39182183 Review.
-
Investigational therapies for Ewing sarcoma: a search without a clear finding.Expert Opin Investig Drugs. 2016 Jun;25(6):679-86. doi: 10.1517/13543784.2016.1168398. Epub 2016 Apr 7. Expert Opin Investig Drugs. 2016. PMID: 26988130 Review.
Cited by
-
Targeting metastasis in paediatric bone sarcomas.Mol Cancer. 2025 May 29;24(1):153. doi: 10.1186/s12943-025-02365-z. Mol Cancer. 2025. PMID: 40442778 Free PMC article. Review.
-
Current Landscape of Immunotherapy for Advanced Sarcoma.Cancers (Basel). 2023 Apr 13;15(8):2287. doi: 10.3390/cancers15082287. Cancers (Basel). 2023. PMID: 37190214 Free PMC article. Review.
-
TCR-transgenic T cells and YB-1-based oncolytic virotherapy improve survival in a preclinical Ewing sarcoma xenograft mouse model.Front Immunol. 2024 Jan 22;15:1330868. doi: 10.3389/fimmu.2024.1330868. eCollection 2024. Front Immunol. 2024. PMID: 38318175 Free PMC article.
-
Suicide gene therapy targeting ewing sarcoma via an ewing-specific GGAA promoter.Sci Rep. 2025 Aug 8;15(1):29020. doi: 10.1038/s41598-025-14945-6. Sci Rep. 2025. PMID: 40781473 Free PMC article.
-
Receptor Tyrosine Kinase Inhibitors for the Treatment of Recurrent and Unresectable Bone Sarcomas.Int J Mol Sci. 2022 Nov 9;23(22):13784. doi: 10.3390/ijms232213784. Int J Mol Sci. 2022. PMID: 36430263 Free PMC article. Review.
References
-
- van Maldegem AM, Bhosale A, Gelderblom HJ, Hogendoorn PC, Hassan AB. Comprehensive analysis of published phase I/II clinical trials between 1990–2010 in osteosarcoma and Ewing sarcoma confirms limited outcomes and need for translational investment. Clin Sarcoma Res. 2012;2(1):1990‐2010. - PMC - PubMed
-
- Gaspar N, Desandes E, Orbach D, et al. Évolution de la prise en charge des sarcomes de l’enfant et de l’adolescent. Oncologie. 2016;18(4):216‐229.
-
- Subbiah V, Anderson P, Lazar AJ, Burdett E, Raymond K, Ludwig JA. Ewing’s sarcoma: standard and experimental treatment options. Curr Treat Options Oncol. 2009;10(1–2):126‐140. - PubMed
-
- Gaspar N, Hawkins DS, Dirksen U, et al. Ewing sarcoma: current management and future approaches through collaboration. J Clin Oncol. 2015;33(27):3036‐3046. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

