Systematic review of phase-I/II trials enrolling refractory and recurrent Ewing sarcoma: Actual knowledge and future directions to optimize the research
- PMID: 33452711
- PMCID: PMC7940237
- DOI: 10.1002/cam4.3712
Systematic review of phase-I/II trials enrolling refractory and recurrent Ewing sarcoma: Actual knowledge and future directions to optimize the research
Abstract
Background: Optimal Phase-II design to evaluate new therapies in refractory/relapsed Ewing sarcomas (ES) remains imperfectly defined.
Objectives: Recurrent/refractory ES phase-I/II trials analysis to improve trials design.
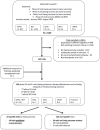
Methods: Comprehensive review of therapeutic trials registered on five databases (who.int/trialsearch, clinicaltrials.gov, clinicaltrialsregister.eu, e-cancer.fr, and umin.ac.jp) and/or published in PubMed/ASCO/ESMO websites, between 2005 and 2018, using the criterion: (Ewing sarcoma OR bone sarcoma OR sarcoma) AND (Phase-I or Phase-II).
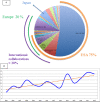
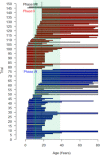
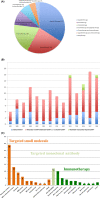
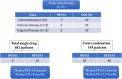
Results: The 146 trials identified (77 phase-I/II, 67 phase-II, and 2 phase-II/III) tested targeted (34%), chemo- (23%), immune therapies (19%), or combined therapies (24%). Twenty-three trials were ES specific and 48 had a specific ES stratum. Usually multicentric (88%), few trials were international (30%). Inclusion criteria cover the recurrent ES age range for only 12% of trials and allowed only accrual of measurable diseases (RECIST criteria). Single-arm design was the most frequent (88%) testing mainly single drugs (61%), only 5% were randomized. Primary efficacy outcome was response rate (RR=CR+PR; Complete+Partial response) (n = 116/146; 79%), rarely progression-free or overall survival (16% PFS and 3% OS). H0 and H1 hypotheses were variable (3%-25% and 20%-50%, respectively). The 62 published trials enrolled 827 ES patients. RR was poor (10%; 15 CR=1.7%, 68 PR=8.3%). Stable disease was the best response for 186 patients (25%). Median PFS/OS was of 1.9 (range 1.3-14.7) and 7.6 months (5-30), respectively. Eleven (18%) published trials were considered positive, with median RR/PFS/OS of 15% (7%-30%), 4.5 (1.3-10), and 16.6 months (6.9-30), respectively.
Conclusion: This review supports the need to develop the international randomized phase-II trials across all age ranges with PFS as primary endpoint.
Keywords: Ewing sarcoma; new cancer therapies; phase-I/II trials; trial design.
© 2021 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures
References
-
- van Maldegem AM, Bhosale A, Gelderblom HJ, Hogendoorn PC, Hassan AB. Comprehensive analysis of published phase I/II clinical trials between 1990–2010 in osteosarcoma and Ewing sarcoma confirms limited outcomes and need for translational investment. Clin Sarcoma Res. 2012;2(1):1990‐2010. - PMC - PubMed
-
- Gaspar N, Desandes E, Orbach D, et al. Évolution de la prise en charge des sarcomes de l’enfant et de l’adolescent. Oncologie. 2016;18(4):216‐229.
-
- Subbiah V, Anderson P, Lazar AJ, Burdett E, Raymond K, Ludwig JA. Ewing’s sarcoma: standard and experimental treatment options. Curr Treat Options Oncol. 2009;10(1–2):126‐140. - PubMed
-
- Gaspar N, Hawkins DS, Dirksen U, et al. Ewing sarcoma: current management and future approaches through collaboration. J Clin Oncol. 2015;33(27):3036‐3046. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

