Presence and short-term persistence of SARS-CoV-2 neutralizing antibodies in COVID-19 convalescent plasma donors
- PMID: 33452828
- PMCID: PMC8014809
- DOI: 10.1111/trf.16261
Presence and short-term persistence of SARS-CoV-2 neutralizing antibodies in COVID-19 convalescent plasma donors
Abstract
Background: In March 2020, the Food and Drug Administration (FDA) approved use of COVID-19 convalescent plasma (CCP) as an investigational new drug for treatment of COVID-19. Since then, collection of CCP from COVID-19-recovered patients has been implemented in donor centers nationwide. Children's Hospital Colorado rapidly put into practice a CCP collection protocol, necessitating development and implementation of assays to evaluate SARS-CoV-2 antibodies in CCP units.
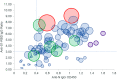
Study design and methods: We evaluated 87 units of CCP collected from 36 donors over two to four sequential donations using both antigen-binding assays for SARS-CoV-2 nucleoprotein and spike antigens and a live virus focus reduction neutralization test (FRNT50 ).
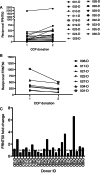
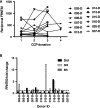
Results: Our data show that the majority of donors (83%) had a FRNT50 titer of at least 80, and 61% had a titer of at least 160, which met the FDA's criteria for acceptable CCP units. Additionally, our data indicate that analysis of antibodies to a single SARS-CoV-2 antigen is likely to miss a percentage of seroconverters; however, these individuals tend to have neutralizing antibody titers of less than 80. There was considerable variability in the short-term, sustained antibody response, measured by neutralizing antibody titers, among our donor population.
Conclusion: The correlation of neutralizing activity and antigen-binding assays is necessary to qualify CCP for therapeutic use. Since SARS-CoV-2 antibody levels decline in a percentage of donors, and such a decline is not detectable by current qualitative assays implemented in many laboratories, robust, quantitative assays are necessary to evaluate CCP units best suited for therapeutic infusion in COVID-19 patients.
Keywords: FFP transfusion; Regulatory and QA; blood component preparations.
© 2021 AABB.
Conflict of interest statement
K.A. provides consulting for Terumo BCT. The other authors declare no potential conflict of interest.
Figures


References
-
- U.S. Food & Drug Administration . Recommendations for investigational COVID‐19 convalescent plasma. https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-in.... Published November 16, 2020. Accessed 02 12, 2021.
-
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research . Investigational COVID‐19 convalescent plasma: guidance for industry. https://www.fda.gov/media/136798/download. Document issued November 16, 2020. Accessed 08 23, 2020.
-
- U.S. Food & Drug Administration . FDA news release: FDA issues emergency use authorization for convalescent plasma as potential promising COVID‐19 treatment, another achievement in administration's fight against pandemic. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency.... Released August 23, 2020. Accessed 03 30, 2020.
-
- U.S. Food & Drug Administration . CFR – code of federal regulations title 21. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?f.... Page current as of April 1, 2020. Accessed 11 10, 2020.
-
- Ciszewski TS, Ralston S, Acteson D, Wasi S, Strong SJ. Protein levels and plasmapheresis intensity. Transfus Med. 1993;3(1):59–65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

