Tissue-Resident Memory CD8+ T Cells From Skin Differentiate Psoriatic Arthritis From Psoriasis
- PMID: 33452865
- PMCID: PMC8362143
- DOI: 10.1002/art.41652
Tissue-Resident Memory CD8+ T Cells From Skin Differentiate Psoriatic Arthritis From Psoriasis
Abstract
Objective: To compare immune cell phenotype and function in psoriatic arthritis (PsA) versus psoriasis in order to better understand the pathogenesis of PsA.
Methods: In-depth immunophenotyping of different T cell and dendritic cell subsets was performed in patients with PsA, psoriasis, or axial spondyloarthritis and healthy controls. Subsequently, we analyzed cells from peripheral blood, synovial fluid (SF), and skin biopsy specimens using flow cytometry, along with high-throughput transcriptome analyses and functional assays on the specific cell populations that appeared to differentiate PsA from psoriasis.
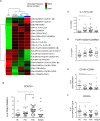
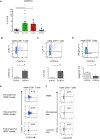
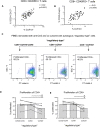
Results: Compared to healthy controls, the peripheral blood of patients with PsA was characterized by an increase in regulatory CD4+ T cells and interleukin-17A (IL-17A) and IL-22 coproducing CD8+ T cells. One population specifically differentiated PsA from psoriasis: i.e., CD8+CCR10+ T cells were enriched in PsA. CD8+CCR10+ T cells expressed high levels of DNAX accessory molecule 1 and were effector memory cells that coexpressed skin-homing receptors CCR4 and cutaneous lymphocyte antigen. CD8+CCR10+ T cells were detected under inflammatory and homeostatic conditions in skin, but were not enriched in SF. Gene profiling further revealed that CD8+CCR10+ T cells expressed GATA3, FOXP3, and core transcriptional signature of tissue-resident memory T cells, including CD103. Specific genes, including RORC, IFNAR1, and ERAP1, were up-regulated in PsA compared to psoriasis. CD8+CCR10+ T cells were endowed with a Tc2/22-like cytokine profile, lacked cytotoxic potential, and displayed overall regulatory function.
Conclusion: Tissue-resident memory CD8+ T cells derived from the skin are enhanced in the circulation of patients with PsA compared to patients with psoriasis alone. This may indicate that aberrances in cutaneous tissue homeostasis contribute to arthritis development.
© 2021 The Authors. Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures



References
-
- Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet 2018;391:2273–84. - PubMed
-
- Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med 2017;376:957–70. - PubMed
-
- Sakkas LI, Bogdanos DP. Are psoriasis and psoriatic arthritis the same disease? The IL‐23/IL‐17 axis data [review]. Autoimmun Rev 2017;16:10–5. - PubMed
-
- Boehncke WH. Psoriasis and psoriatic arthritis: flip sides of the coin? Acta Derm Venereol 2016;96:436–41. - PubMed
-
- McGonagle D, Aydin SZ, Gül A, Mahr A, Direskeneli H. ’MHC‐I‐opathy’‐unified concept for spondyloarthritis and Behçet disease [review]. Nat Rev Rheumatol 2015;11:731–40. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

