Evaluation of two rapid antigen tests to detect SARS-CoV-2 in a hospital setting
- PMID: 33452927
- PMCID: PMC7811156
- DOI: 10.1007/s00430-020-00698-8
Evaluation of two rapid antigen tests to detect SARS-CoV-2 in a hospital setting
Abstract
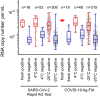
Successful containment strategies for the SARS-CoV-2 pandemic will depend on reliable diagnostic assays. Point-of-care antigen tests (POCT) may provide an alternative to time-consuming PCR tests to rapidly screen for acute infections on site. Here, we evaluated two SARS-CoV-2 antigen tests: the STANDARD™ F COVID-19 Ag FIA (FIA) and the SARS-CoV-2 Rapid Antigen Test (RAT). For diagnostic assessment, we used a large set of PCR-positive and PCR-negative respiratory swabs from asymptomatic and symptomatic patients and health care workers in the setting of two University Hospitals in Munich, Germany, i.e. emergency rooms, patient care units or employee test centers. For FIA, overall clinical sensitivity and specificity were 45.4% (n = 381) and 97.8% (n = 360), respectively, and for RAT, 50.3% (n = 445) and 97.7% (n = 386), respectively. For primary diagnosis of asymptomatic and symptomatic individuals, diagnostic sensitivities were 60.9% (FIA) (n = 189) and 64.5% (RAT) (n = 256). This questions these tests' utility for the reliable detection of acute SARS-CoV-2-infected individuals, in particular in high-risk settings. We support the proposal that convincing high-quality outcome data on the impact of false-negative and false-positive antigen test results need to be obtained in a POCT setting. Moreover, the efficacy of alternative testing strategies to complement PCR assays must be evaluated by independent laboratories, prior to widespread implementation in national and international test strategies.
Keywords: COVID-19 point-of-care; Diagnostic test; SARS-CoV-2 antigen test; Sensitivity; Specificity.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Centers for Disease Control and Prevention (CDC). Information for laboratories about coronavirus (COVID-19). Atlanta: CDC. https://www.cdc.gov/coronavirus/2019-ncov/lab/index.html. Accessed 16 Apr 2020
-
- Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T, Brünink S, Schneider J, Schmidt ML, Mulders DG, Haagmans BL, van der Veer B, van den Brink S, Wijsman L, Goderski G, Romette JL, Ellis J, Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MP, Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020 doi: 10.2807/1560-7917.Es.2020.25.3.2000045. - DOI - PMC - PubMed
-
- Konrad R, Eberle U, Dangel A, Treis B, Berger A, Bengs K, Fingerle V, Liebl B, Ackermann N, Sing A. Rapid establishment of laboratory diagnostics for the novel coronavirus SARS-CoV-2 in Bavaria, Germany, February 2020. Euro Surveill. 2020 doi: 10.2807/1560-7917.Es.2020.25.9.2000173. - DOI - PMC - PubMed
-
- Muenchhoff M, Mairhofer H, Nitschko H, Grzimek-Koschewa N, Hoffmann D, Berger A, Rabenau H, Widera M, Ackermann N, Konrad R, Zange S, Graf A, Krebs S, Blum H, Sing A, Liebl B, Wölfel R, Ciesek S, Drosten C, Protzer U, Boehm S, Keppler OT. Multicentre comparison of quantitative PCR-based assays to detect SARS-CoV-2, Germany, March 2020. Euro Surveill. 2020 doi: 10.2807/1560-7917.Es.2020.25.24.2001057. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

