Developments in the Dehydrogenative Electrochemical Synthesis of 3,3',5,5'-Tetramethyl-2,2'-biphenol
- PMID: 33453091
- PMCID: PMC8248109
- DOI: 10.1002/chem.202005197
Developments in the Dehydrogenative Electrochemical Synthesis of 3,3',5,5'-Tetramethyl-2,2'-biphenol
Abstract
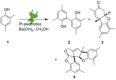
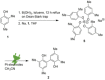
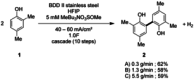
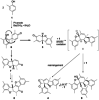
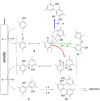
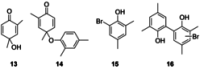
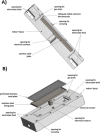
The symmetric biphenol 3,3',5,5'-tetramethyl-2,2'-biphenol is a well-known ligand building block and is used in transition-metal catalysis. In the literature, there are several synthetic routes for the preparation of this exceptional molecule. Herein, the focus is on the sustainable electrochemical synthesis of 3,3',5,5'-tetramethyl-2,2'-biphenol. A brief overview of the developmental history of this inconspicuous molecule, which is of great interest for technical applications, but has many challenges for its synthesis, is provided. The electro-organic method is a powerful, sustainable, and efficient alternative to conventional synthesis to obtain this symmetric biphenol up to the kilogram scale. Another section of this article is devoted to different process management strategies in batch-type and flow electrolysis and their respective advantages.
Keywords: C−C coupling; electrochemistry; oxidation; polycycles; sustainable chemistry.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- None
-
- Bringmann G., Gulder T., Gulder T. A. M., Breuning M., Chem. Rev. 2011, 111, 563–639; - PubMed
-
- von Nussbaum F., Brands M., Hinzen B., Weigand S., Häbich D., Angew. Chem. Int. Ed. 2006, 45, 5072–5129; - PubMed
- Angew. Chem. 2006, 118, 5194–5254.
-
- Bringmann G., Price Mortimer A. J., Keller P. A., Gresser M. J., Garner J., Breuning M., Angew. Chem. Int. Ed. 2005, 44, 5384–5427; - PubMed
- Angew. Chem. 2005, 117, 5518–5563.
-
- Selt M., Mentizi S., Schollmeyer D., Franke R., Waldvogel S. R., Synlett 2019, 30, 2062–2067.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

