A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial
- PMID: 33453783
- PMCID: PMC9194961
- DOI: 10.1016/S0140-6736(20)32514-9
A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial
Abstract
Background: Management of type 1 diabetes is challenging. We compared outcomes using a commercially available hybrid closed-loop system versus a new investigational system with features potentially useful for adolescents and young adults with type 1 diabetes.
Methods: In this multinational, randomised, crossover trial (Fuzzy Logic Automated Insulin Regulation [FLAIR]), individuals aged 14-29 years old, with a clinical diagnosis of type 1 diabetes with a duration of at least 1 year, using either an insulin pump or multiple daily insulin injections, and glycated haemoglobin (HbA1c) levels of 7·0-11·0% (53-97 mmol/mol) were recruited from seven academic-based endocrinology practices, four in the USA, and one each in Germany, Israel, and Slovenia. After a run-in period to teach participants how to use the study pump and continuous glucose monitor, participants were randomly assigned (1:1) using a computer-generated sequence, with a permuted block design (block sizes of two and four), stratified by baseline HbA1c and use of a personal MiniMed 670G system (Medtronic) at enrolment, to either use of a MiniMed 670G hybrid closed-loop system (670G) or the investigational advanced hybrid closed-loop system (Medtronic) for the first 12-week period, and then participants were crossed over with no washout period, to the other group for use for another 12 weeks. Masking was not possible due to the nature of the systems used. The coprimary outcomes, measured with continuous glucose monitoring, were proportion of time that glucose levels were above 180 mg/dL (>10·0 mmol/L) during 0600 h to 2359 h (ie, daytime), tested for superiority, and proportion of time that glucose levels were below 54 mg/dL (<3·0 mmol/L) calculated over a full 24-h period, tested for non-inferiority (non-inferiority margin 2%). Analysis was by intention to treat. Safety was assessed in all participants randomly assigned to treatment. This trial is registered with ClinicalTrials.gov, NCT03040414, and is now complete.
Findings: Between June 3 and Aug 22, 2019, 113 individuals were enrolled into the trial. Mean age was 19 years (SD 4) and 70 (62%) of 113 participants were female. Mean proportion of time with daytime glucose levels above 180 mg/dL (>10·0 mmol/L) was 42% (SD 13) at baseline, 37% (9) during use of the 670G system, and 34% (9) during use of the advanced hybrid closed-loop system (mean difference [advanced hybrid closed-loop system minus 670G system] -3·00% [95% CI -3·97 to -2·04]; p<0·0001). Mean 24-h proportion of time with glucose levels below 54 mg/dL (<3·0 mmol/L) was 0·46% (SD 0·42) at baseline, 0·50% (0·35) during use of the 670G system, and 0·46% (0·33) during use of the advanced hybrid closed-loop system (mean difference [advanced hybrid closed-loop system minus 670G system] -0·06% [95% CI -0·11 to -0·02]; p<0·0001 for non-inferiority). One severe hypoglycaemic event occurred in the advanced hybrid closed-loop system group, determined to be unrelated to study treatment, and none occurred in the 670G group.
Interpretation: Hyperglycaemia was reduced without increasing hypoglycaemia in adolescents and young adults with type 1 diabetes using the investigational advanced hybrid closed-loop system compared with the commercially available MiniMed 670G system. Testing an advanced hybrid closed-loop system in populations that are underserved due to socioeconomic factors and testing during pregnancy and in individuals with impaired awareness of hypoglycaemia would advance the effective use of this technology FUNDING: National Institute of Diabetes and Digestive and Kidney Diseases.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures


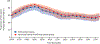
References
-
- Beck RW, Bergenstal RM, Laffel LM, Pickup JC. Advances in technology for management of type 1 diabetes. Lancet 2019; 394(10205): 1265–73. - PubMed
-
- Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a Hybrid Closed-Loop Insulin Delivery System in Patients With Type 1 Diabetes. JAMA 2016; 316(13): 1407–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

