Post-translational control of the long and winding road to cholesterol
- PMID: 33453997
- PMCID: PMC7762936
- DOI: 10.1074/jbc.REV120.010723
Post-translational control of the long and winding road to cholesterol
Abstract
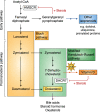
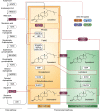
The synthesis of cholesterol requires more than 20 enzymes, many of which are intricately regulated. Post-translational control of these enzymes provides a rapid means for modifying flux through the pathway. So far, several enzymes have been shown to be rapidly degraded through the ubiquitin-proteasome pathway in response to cholesterol and other sterol intermediates. Additionally, several enzymes have their activity altered through phosphorylation mechanisms. Most work has focused on the two rate-limiting enzymes: 3-hydroxy-3-methylglutaryl CoA reductase and squalene monooxygenase. Here, we review current literature in the area to define some common themes in the regulation of the entire cholesterol synthesis pathway. We highlight the rich variety of inputs controlling each enzyme, discuss the interplay that exists between regulatory mechanisms, and summarize findings that reveal an intricately coordinated network of regulation along the cholesterol synthesis pathway. We provide a roadmap for future research into the post-translational control of cholesterol synthesis, and no doubt the road ahead will reveal further twists and turns for this fascinating pathway crucial for human health and disease.
Keywords: E3 ubiquitin ligase; HMGCR; SM; acetylation; cholesterol; cholesterol metabolism; cholesterol regulation; cholesterol synthesis; phosphorylation; post-translational modification; post-translational modification (PTM); post-translational regulation; proteasome; protein degradation; ubiquitin ligase; ubiquitination; ubiquitylation (ubiquitination).
Copyright © 2020 © 2020 Sharpe et al. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

Similar articles
-
Differential use of E2 ubiquitin conjugating enzymes for regulated degradation of the rate-limiting enzymes HMGCR and SQLE in cholesterol biosynthesis.Atherosclerosis. 2019 Feb;281:137-142. doi: 10.1016/j.atherosclerosis.2018.12.008. Epub 2018 Dec 23. Atherosclerosis. 2019. PMID: 30658189
-
The UPS and downs of cholesterol homeostasis.Trends Biochem Sci. 2014 Nov;39(11):527-35. doi: 10.1016/j.tibs.2014.08.008. Epub 2014 Sep 11. Trends Biochem Sci. 2014. PMID: 25220377 Review.
-
Navigating the Shallows and Rapids of Cholesterol Synthesis Downstream of HMGCR.J Nutr Sci Vitaminol (Tokyo). 2015;61 Suppl:S154-6. doi: 10.3177/jnsv.61.S154. J Nutr Sci Vitaminol (Tokyo). 2015. PMID: 26598836 Review.
-
Protein turnover regulated by cholesterol.Curr Opin Lipidol. 2012 Feb;23(1):76-7. doi: 10.1097/MOL.0b013e32834f42b3. Curr Opin Lipidol. 2012. PMID: 22241328 No abstract available.
-
Controlling cholesterol synthesis beyond 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR).J Biol Chem. 2013 Jun 28;288(26):18707-15. doi: 10.1074/jbc.R113.479808. Epub 2013 May 21. J Biol Chem. 2013. PMID: 23696639 Free PMC article. Review.
Cited by
-
Exploration on the Mechanism of Ubiquitin Proteasome System in Cerebral Stroke.Front Aging Neurosci. 2022 Apr 7;14:814463. doi: 10.3389/fnagi.2022.814463. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35462700 Free PMC article. Review.
-
Lipid-Lowering Drugs and Pulmonary Vascular Disease: A Mendelian Randomization Study.Pulm Circ. 2025 Jan 22;15(1):e70043. doi: 10.1002/pul2.70043. eCollection 2025 Jan. Pulm Circ. 2025. PMID: 39850014 Free PMC article.
-
Chronic Aripiprazole and Trazodone Polypharmacy Effects on Systemic and Brain Cholesterol Biosynthesis.Biomolecules. 2023 Aug 28;13(9):1321. doi: 10.3390/biom13091321. Biomolecules. 2023. PMID: 37759721 Free PMC article.
-
Regulation of cholesterol homeostasis in health and diseases: from mechanisms to targeted therapeutics.Signal Transduct Target Ther. 2022 Aug 2;7(1):265. doi: 10.1038/s41392-022-01125-5. Signal Transduct Target Ther. 2022. PMID: 35918332 Free PMC article. Review.
-
Enzymes in the Cholesterol Synthesis Pathway: Interactomics in the Cancer Context.Biomedicines. 2021 Jul 26;9(8):895. doi: 10.3390/biomedicines9080895. Biomedicines. 2021. PMID: 34440098 Free PMC article.
References
-
- Wang N., Fulcher J., Abeysuriya N., Park L., Kumar S., Di Tanna G. L., Wilcox I., Keech A., Rodgers A., and Lal S. (2020) Intensive LDL cholesterol-lowering treatment beyond current recommendations for the prevention of major vascular events: a systematic review and meta-analysis of randomised trials including 327 037 participants. Lancet Diabetes Endocrinol. 8, 36–49 10.1016/S2213-8587(19)30388-2 - DOI - PubMed
-
- Brown A. J., and Sharpe L. J. (2016) Cholesterol synthesis. In Biochemistry of Lipids, Lipoproteins, and Membranes, 6th Ed., pp. 327–358, Elsevier Science Publishing Co., Inc., New York
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

