The HRDC domain oppositely modulates the unwinding activity of E. coli RecQ helicase on duplex DNA and G-quadruplex
- PMID: 33454004
- PMCID: PMC7762929
- DOI: 10.1074/jbc.RA120.015492
The HRDC domain oppositely modulates the unwinding activity of E. coli RecQ helicase on duplex DNA and G-quadruplex
Abstract
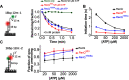
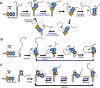
RecQ family helicases are highly conserved from bacteria to humans and have essential roles in maintaining genome stability. Mutations in three human RecQ helicases cause severe diseases with the main features of premature aging and cancer predisposition. Most RecQ helicases shared a conserved domain arrangement which comprises a helicase core, an RecQ C-terminal domain, and an auxiliary element helicase and RNaseD C-terminal (HRDC) domain, the functions of which are poorly understood. In this study, we systematically characterized the roles of the HRDC domain in E. coli RecQ in various DNA transactions by single-molecule FRET. We found that RecQ repetitively unwinds the 3'-partial duplex and fork DNA with a moderate processivity and periodically patrols on the ssDNA in the 5'-partial duplex by translocation. The HRDC domain significantly suppresses RecQ activities in the above transactions. In sharp contrast, the HRDC domain is essential for the deep and long-time unfolding of the G4 DNA structure by RecQ. Based on the observations that the HRDC domain dynamically switches between RecA core- and ssDNA-binding modes after RecQ association with DNA, we proposed a model to explain the modulation mechanism of the HRDC domain. Our findings not only provide new insights into the activities of RecQ on different substrates but also highlight the novel functions of the HRDC domain in DNA metabolisms.
Keywords: DNA repair; E. coli; G-quadruplex; RecQ; helicase; single-molecule; unwinding.
Copyright © 2020 © 2020 Teng et al. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

