Mycobacterium tuberculosis infection up-regulates MFN2 expression to promote NLRP3 inflammasome formation
- PMID: 33454007
- PMCID: PMC7762945
- DOI: 10.1074/jbc.RA120.014077
Mycobacterium tuberculosis infection up-regulates MFN2 expression to promote NLRP3 inflammasome formation
Abstract
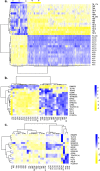
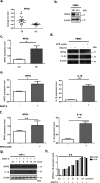
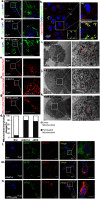
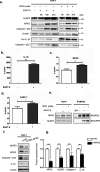
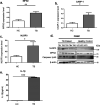
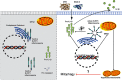
Tuberculosis (TB), caused by the infection of Mycobacterium tuberculosis (MTB), is one of the leading causes of death worldwide, especially in children. However, the mechanisms by which MTB infects its cellular host, activates an immune response, and triggers inflammation remain unknown. Mitochondria play important roles in the initiation and activation of the nucleotide-binding oligomerization domain-like receptor with a pyrin domain 3 (NLRP3) inflammasome, where mitochondria-associated endoplasmic reticulum membranes (MAMs) may serve as the platform for inflammasome assembly and activation. Additionally, mitofusin 2 (MFN2) is implicated in the formation of MAMs, but, the roles of mitochondria and MFN2 in MTB infection have not been elucidated. Using mircroarry profiling of TB patients and in vitro MTB stimulation of macrophages, we observed an up-regulation of MFN2 in the peripheral blood mononuclear cells of active TB patients. Furthermore, we found that MTB stimulation by MTB-specific antigen ESAT-6 or lysate of MTB promoted MFN2 interaction with NLRP3 inflammasomes, resulting in the assembly and activation of the inflammasome and, subsequently, IL-1β secretion. These findings suggest that MFN2 and mitochondria play important role in the pathogen-host interaction during MTB infection.
Keywords: Mycobacterium tuberculosis; NLRP3 inflammasome; cytokine; cytokine inflammasome; microarray; microarray mitochondria; mitochondria; mitofusin 2.
Copyright © 2020 © 2020 Xu et al. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests for this work
Figures
Similar articles
-
Repression of BRD4 mitigates NLRP3 inflammasome-mediated pyroptosis in Mycobacterium-infected macrophages by repressing endoplasmic reticulum stress.Tuberculosis (Edinb). 2024 Sep;148:102542. doi: 10.1016/j.tube.2024.102542. Epub 2024 Jul 11. Tuberculosis (Edinb). 2024. PMID: 39024987
-
Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome.Cell Microbiol. 2010 Aug;12(8):1046-63. doi: 10.1111/j.1462-5822.2010.01450.x. Epub 2010 Feb 9. Cell Microbiol. 2010. PMID: 20148899
-
Mitigation of lipopolysaccharide-induced intestinal injury in rats by Chimonanthus nitens Oliv. essential oil via suppression of mitochondrial fusion protein mitofusin 2 (MFN2)-mediated mitochondrial-associated endoplasmic reticulum membranes (MAMs) formation.J Ethnopharmacol. 2025 Jan 30;337(Pt 2):118856. doi: 10.1016/j.jep.2024.118856. Epub 2024 Sep 25. J Ethnopharmacol. 2025. PMID: 39332614
-
Exploring the Use of Medicinal Plants and Their Bioactive Derivatives as Alveolar NLRP3 Inflammasome Regulators during Mycobacterium tuberculosis Infection.Int J Mol Sci. 2021 Aug 31;22(17):9497. doi: 10.3390/ijms22179497. Int J Mol Sci. 2021. PMID: 34502407 Free PMC article. Review.
-
Interaction of Mycobacteria With Host Cell Inflammasomes.Front Immunol. 2022 Feb 14;13:791136. doi: 10.3389/fimmu.2022.791136. eCollection 2022. Front Immunol. 2022. PMID: 35237260 Free PMC article. Review.
Cited by
-
The Role of NLRP3 Inflammasome in Alzheimer's Disease and Potential Therapeutic Targets.Front Pharmacol. 2022 Feb 16;13:845185. doi: 10.3389/fphar.2022.845185. eCollection 2022. Front Pharmacol. 2022. PMID: 35250595 Free PMC article. Review.
-
The complex interplay between endoplasmic reticulum stress and the NLRP3 inflammasome: a potential therapeutic target for inflammatory disorders.Clin Transl Immunology. 2021 Feb 11;10(2):e1247. doi: 10.1002/cti2.1247. eCollection 2021. Clin Transl Immunology. 2021. PMID: 33614031 Free PMC article. Review.
-
Large yellow croaker (Larimichthys crocea) mitofusin 2 inhibits type I IFN responses by degrading MAVS via enhanced K48-linked ubiquitination.Mar Life Sci Technol. 2023 Aug 18;5(3):359-372. doi: 10.1007/s42995-023-00189-8. eCollection 2023 Aug. Mar Life Sci Technol. 2023. PMID: 37637256 Free PMC article.
-
Structure and Function of Mitochondria-Associated Endoplasmic Reticulum Membranes (MAMs) and Their Role in Cardiovascular Diseases.Oxid Med Cell Longev. 2021 Jul 11;2021:4578809. doi: 10.1155/2021/4578809. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34336092 Free PMC article. Review.
-
The role of mitochondria-associated membranes mediated ROS on NLRP3 inflammasome in cardiovascular diseases.Front Cardiovasc Med. 2022 Dec 14;9:1059576. doi: 10.3389/fcvm.2022.1059576. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36588561 Free PMC article. Review.
References
-
- WHO (2019) Global Tuberculosis Report, World Health Organization
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

