A novel method produces native light-harvesting complex II aggregates from the photosynthetic membrane revealing their role in nonphotochemical quenching
- PMID: 33454016
- PMCID: PMC7762933
- DOI: 10.1074/jbc.RA120.016181
A novel method produces native light-harvesting complex II aggregates from the photosynthetic membrane revealing their role in nonphotochemical quenching
Abstract
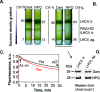
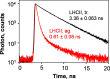
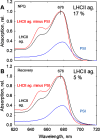
Nonphotochemical quenching (NPQ) is a mechanism of regulating light harvesting that protects the photosynthetic apparatus from photodamage by dissipating excess absorbed excitation energy as heat. In higher plants, the major light-harvesting antenna complex (LHCII) of photosystem (PS) II is directly involved in NPQ. The aggregation of LHCII is proposed to be involved in quenching. However, the lack of success in isolating native LHCII aggregates has limited the direct interrogation of this process. The isolation of LHCII in its native state from thylakoid membranes has been problematic because of the use of detergent, which tends to dissociate loosely bound proteins, and the abundance of pigment-protein complexes (e.g. PSI and PSII) embedded in the photosynthetic membrane, which hinders the preparation of aggregated LHCII. Here, we used a novel purification method employing detergent and amphipols to entrap LHCII in its natural states. To enrich the photosynthetic membrane with the major LHCII, we used Arabidopsis thaliana plants lacking the PSII minor antenna complexes (NoM), treated with lincomycin to inhibit the synthesis of PSI and PSII core proteins. Using sucrose density gradients, we succeeded in isolating the trimeric and aggregated forms of LHCII antenna. Violaxanthin- and zeaxanthin-enriched complexes were investigated in dark-adapted, NPQ, and dark recovery states. Zeaxanthin-enriched antenna complexes showed the greatest amount of aggregated LHCII. Notably, the amount of aggregated LHCII decreased upon relaxation of NPQ. Employing this novel preparative method, we obtained a direct evidence for the role of in vivo LHCII aggregation in NPQ.
Keywords: LHCII aggregate; LHCII trimer; NPQ; NoM; amphipol A8-35; chlorophyll; fluorescence; light-harvesting complex (antenna complex); lincomycin; nonphotochemical quenching; photosystem II; plant biochemistry.
Copyright © 2020 © 2020 Shukla et al. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



Similar articles
-
The xanthophyll cycle affects reversible interactions between PsbS and light-harvesting complex II to control non-photochemical quenching.Nat Plants. 2017 Jan 30;3:16225. doi: 10.1038/nplants.2016.225. Nat Plants. 2017. PMID: 28134919
-
The causes of altered chlorophyll fluorescence quenching induction in the Arabidopsis mutant lacking all minor antenna complexes.Biochim Biophys Acta Bioenerg. 2018 Sep;1859(9):666-675. doi: 10.1016/j.bbabio.2018.03.005. Epub 2018 Mar 13. Biochim Biophys Acta Bioenerg. 2018. PMID: 29548769
-
Higher plant photosystem II light-harvesting antenna, not the reaction center, determines the excited-state lifetime-both the maximum and the nonphotochemically quenched.Biophys J. 2012 Jun 20;102(12):2761-71. doi: 10.1016/j.bpj.2012.05.004. Epub 2012 Jun 19. Biophys J. 2012. PMID: 22735526 Free PMC article.
-
Light harvesting control in plants.FEBS Lett. 2018 Sep;592(18):3030-3039. doi: 10.1002/1873-3468.13111. Epub 2018 Jun 4. FEBS Lett. 2018. PMID: 29797317 Review.
-
Energy-Dependent Non-Photochemical Quenching: PsbS, LhcSR, and Other Players.Biochemistry (Mosc). 2025 Jan;90(1):44-60. doi: 10.1134/S000629792460371X. Biochemistry (Mosc). 2025. PMID: 40058973 Review.
Cited by
-
Expression of the Arabidopsis Mg-chelatase H subunit alleviates iron deficiency-induced stress in transgenic rice.Front Plant Sci. 2023 Mar 2;14:1098808. doi: 10.3389/fpls.2023.1098808. eCollection 2023. Front Plant Sci. 2023. PMID: 36938029 Free PMC article.
-
Identification of distinct pH- and zeaxanthin-dependent quenching in LHCSR3 from Chlamydomonas reinhardtii.Elife. 2021 Jan 15;10:e60383. doi: 10.7554/eLife.60383. Elife. 2021. PMID: 33448262 Free PMC article.
-
Photosystem II photoinhibition and photoprotection in a lycophyte, Selaginella martensii.Physiol Plant. 2022 Jan;174(1):e13604. doi: 10.1111/ppl.13604. Epub 2021 Dec 6. Physiol Plant. 2022. PMID: 34811759 Free PMC article.
-
Energetic driving force for LHCII clustering in plant membranes.Sci Adv. 2023 Dec 22;9(51):eadj0807. doi: 10.1126/sciadv.adj0807. Epub 2023 Dec 22. Sci Adv. 2023. PMID: 38134273 Free PMC article.
-
A perspective on the major light-harvesting complex dynamics under the effect of pH, salts, and the photoprotective PsbS protein.Photosynth Res. 2023 Apr;156(1):163-177. doi: 10.1007/s11120-022-00935-6. Epub 2022 Jul 10. Photosynth Res. 2023. PMID: 35816266 Free PMC article. Review.
References
-
- Blankenship R. E. (2014) Molecular Mechanisms of Photosynthesis, John Wiley & Sons, New York
-
- Barber J. (1995) Molecular basis of the vulnerability of photosystem II to damage by light. Funct. Plant Biol. 22, 201–208 10.1071/PP9950201 - DOI
-
- Demmig-Adams B., and Adams W. W. 3rd (1992) Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant Biol. 43, 599–626 10.1146/annurev.pp.43.060192.003123 - DOI
-
- Powles S. B. (1984) Photoinhibition of photosynthesis induced by visible light. Annu. Rev. Plant Physiol. 35, 15–44 10.1146/annurev.pp.35.060184.000311 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

