Antibiotic binding releases autoinhibition of the TipA multidrug-resistance transcriptional regulator
- PMID: 33454020
- PMCID: PMC7762955
- DOI: 10.1074/jbc.RA120.016295
Antibiotic binding releases autoinhibition of the TipA multidrug-resistance transcriptional regulator
Abstract
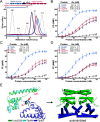
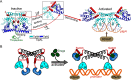
Investigations of bacterial resistance strategies can aid in the development of new antimicrobial drugs as a countermeasure to the increasing worldwide prevalence of bacterial antibiotic resistance. One such strategy involves the TipA class of transcription factors, which constitute minimal autoregulated multidrug resistance (MDR) systems against diverse antibiotics. However, we have insufficient information regarding how antibiotic binding induces transcriptional activation to design molecules that could interfere with this process. To learn more, we determined the crystal structure of SkgA from Caulobacter crescentus as a representative TipA protein. We identified an unexpected spatial orientation and location of the antibiotic-binding TipAS effector domain in the apo state. We observed that the α6-α7 region of the TipAS domain, which is canonically responsible for forming the lid of antibiotic-binding cleft to tightly enclose the bound antibiotic, is involved in the dimeric interface and stabilized via interaction with the DNA-binding domain in the apo state. Further structural and biochemical analyses demonstrated that the unliganded TipAS domain sterically hinders promoter DNA binding but undergoes a remarkable conformational shift upon antibiotic binding to release this autoinhibition via a switch of its α6-α7 region. Hence, the promoters for MDR genes including tipA and RNA polymerases become available for transcription, enabling efficient antibiotic resistance. These insights into the molecular mechanism of activation of TipA proteins advance our understanding of TipA proteins, as well as bacterial MDR systems, and may provide important clues to block bacterial resistance.
Keywords: DNA-binding protein; TipA; activation mechanism; antibiotic resistance; autoinhibition; crystal structure; drug resistance; multidrug resistance (MDR); structure biology; transcription promoter; transcription regulation; transcriptional regulator.
Copyright © 2020 © 2020 Jiang et al. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest with the contents of this article
Figures




Similar articles
-
Structural basis for antibiotic recognition by the TipA class of multidrug-resistance transcriptional regulators.EMBO J. 2003 Apr 15;22(8):1824-34. doi: 10.1093/emboj/cdg181. EMBO J. 2003. PMID: 12682015 Free PMC article.
-
Ligand-induced changes in the Streptomyces lividans TipAL protein imply an alternative mechanism of transcriptional activation for MerR-like proteins.Biochemistry. 2001 Oct 30;40(43):12950-8. doi: 10.1021/bi010328k. Biochemistry. 2001. PMID: 11669632
-
Structural basis and dynamics of multidrug recognition in a minimal bacterial multidrug resistance system.Proc Natl Acad Sci U S A. 2014 Dec 23;111(51):E5498-507. doi: 10.1073/pnas.1412070111. Epub 2014 Dec 8. Proc Natl Acad Sci U S A. 2014. PMID: 25489067 Free PMC article.
-
Protein-protein complexes as targets for drug discovery against infectious diseases.Adv Protein Chem Struct Biol. 2020;121:237-251. doi: 10.1016/bs.apcsb.2019.11.012. Epub 2019 Dec 18. Adv Protein Chem Struct Biol. 2020. PMID: 32312423 Review.
-
Targeting bacterial transcription factors for infection control: opportunities and challenges.Transcription. 2025 Feb;16(1):141-168. doi: 10.1080/21541264.2023.2293523. Epub 2023 Dec 21. Transcription. 2025. PMID: 38126125 Free PMC article. Review.
Cited by
-
Allosteric activation mechanism of DriD, a WYL-domain containing transcription regulator.Commun Biol. 2025 Apr 29;8(1):679. doi: 10.1038/s42003-025-08111-x. Commun Biol. 2025. PMID: 40301632 Free PMC article.
-
Bacterial Transcriptional Regulators: A Road Map for Functional, Structural, and Biophysical Characterization.Int J Mol Sci. 2022 Feb 16;23(4):2179. doi: 10.3390/ijms23042179. Int J Mol Sci. 2022. PMID: 35216300 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

