Genetic diseases of the Kennedy pathways for membrane synthesis
- PMID: 33454021
- PMCID: PMC7762932
- DOI: 10.1074/jbc.REV120.013529
Genetic diseases of the Kennedy pathways for membrane synthesis
Abstract
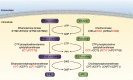
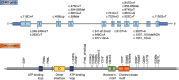
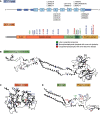
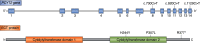
The two branches of the Kennedy pathways (CDP-choline and CDP-ethanolamine) are the predominant pathways responsible for the synthesis of the most abundant phospholipids, phosphatidylcholine and phosphatidylethanolamine, respectively, in mammalian membranes. Recently, hereditary diseases associated with single gene mutations in the Kennedy pathways have been identified. Interestingly, genetic diseases within the same pathway vary greatly, ranging from muscular dystrophy to spastic paraplegia to a childhood blinding disorder to bone deformations. Indeed, different point mutations in the same gene (PCYT1; CCTα) result in at least three distinct diseases. In this review, we will summarize and review the genetic diseases associated with mutations in genes of the Kennedy pathway for phospholipid synthesis. These single-gene disorders provide insight, indeed direct genotype-phenotype relationships, into the biological functions of specific enzymes of the Kennedy pathway. We discuss potential mechanisms of how mutations within the same pathway can cause disparate disease.
Keywords: genetic disease; inherited; lipid; membrane; metabolism; phosphatidylcholine; phosphatidylethanolamine; phospholipid.
Copyright © 2020 © 2020 Tavasoli et al. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

