Trends and strategies to combat viral infections: A review on FDA approved antiviral drugs
- PMID: 33454328
- PMCID: PMC8055758
- DOI: 10.1016/j.ijbiomac.2021.01.076
Trends and strategies to combat viral infections: A review on FDA approved antiviral drugs
Abstract
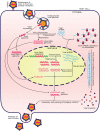
The infectious microscopic viruses invade living cells to reproduce themselves, and causes chronic infections such as HIV/AIDS, hepatitis B and C, flu, etc. in humans which may lead to death if not treated. Different strategies have been utilized to develop new and superior antiviral drugs to counter the viral infections. The FDA approval of HIV nucleoside reverse transcriptase inhibitor, zidovudine in 1987 boosted the development of antiviral agents against different viruses. Currently, there are a number of combination drugs developed against various viral infections to arrest the activity of same or different viral macromolecules at multiple stages of its life cycle; among which majority are targeted to interfere with the replication of viral genome. Besides these, other type of antiviral molecules includes entry inhibitors, integrase inhibitors, protease inhibitors, interferons, immunomodulators, etc. The antiviral drugs can be toxic to human cells, particularly in case of administration of combination drugs, and on the other hand viruses can grow resistant to the antiviral drugs. Furthermore, emergence of new viruses like Ebola, coronaviruses (SARS-CoV, SARS-CoV-2) emphasizes the need for more innovative strategies to develop better antiviral drugs to fight the existing and the emerging viral infections. Hence, we reviewed the strategic enhancements in developing antiviral drugs for the treatment of different viral infections over the years.
Keywords: Antiviral drugs; Combination therapies; Entry inhibitors; NNRTIs; NRTIs; Protease inhibitors; Virus pandemics.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no conflict of interest.
Figures
References
-
- H. Lodish, A. Berk, S.L. Zipursky, P. Matsudaira, D. Baltimore, J. Darnell, Molecular Cell Biology. 4 ed., W.H. Freeman & Co. Ltd, New York, 2000.
-
- Greub G., Ledergerber B., Battegay M., Grob P., Perrin L., Furrer H., Burgisser P., Erb P., Boggian K., Piffaretti J.C., Hirschel B., Janin P., Francioli P., Flepp M., Telenti A. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV cohort study. Lancet. 2000;356(9244):1800–1805. doi: 10.1016/s0140-6736(00)03232-3. - DOI - PubMed
-
- CDC, HIV/AIDs Surveillance Report, US Department of Health and Human Services, Public Health Service . Center for Infectious Diseases; Division of HIV/AIDS: 1990. Centers for Disease Control; pp. 1–18.
-
- A.S. Evans, Viral Infections of Humans: Epidemiology and Control, 3 ed., Springer Science & Business Media2013.
-
- Soderstrom K. In: xPharm: The Comprehensive Pharmacology Reference. Enna S.J., Bylund D.B., editors. Elsevier; New York: 2007. Viral replication; pp. 1–5.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

