From biomechanics to pathology: predicting axonal injury from patterns of strain after traumatic brain injury
- PMID: 33454735
- PMCID: PMC7990483
- DOI: 10.1093/brain/awaa336
From biomechanics to pathology: predicting axonal injury from patterns of strain after traumatic brain injury
Abstract
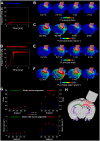
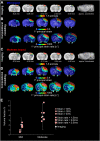
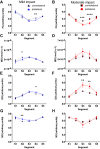
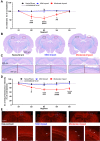
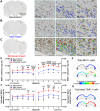
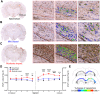
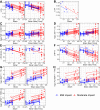
The relationship between biomechanical forces and neuropathology is key to understanding traumatic brain injury. White matter tracts are damaged by high shear forces during impact, resulting in axonal injury, a key determinant of long-term clinical outcomes. However, the relationship between biomechanical forces and patterns of white matter injuries, associated with persistent diffusion MRI abnormalities, is poorly understood. This limits the ability to predict the severity of head injuries and the design of appropriate protection. Our previously developed human finite element model of head injury predicted the location of post-traumatic neurodegeneration. A similar rat model now allows us to experimentally test whether strain patterns calculated by the model predicts in vivo MRI and histology changes. Using a controlled cortical impact, mild and moderate injuries (1 and 2 mm) were performed. Focal and axonal injuries were quantified with volumetric and diffusion 9.4 T MRI at 2 weeks post injury. Detailed analysis of the corpus callosum was conducted using multi-shell diffusion MRI and histopathology. Microglia and astrocyte density, including process parameters, along with white matter structural integrity and neurofilament expression were determined by quantitative immunohistochemistry. Linear mixed effects regression analyses for strain and strain rate with the employed outcome measures were used to ascertain how well immediate biomechanics could explain MRI and histology changes. The spatial pattern of mechanical strain and strain rate in the injured cortex shows good agreement with the probability maps of focal lesions derived from volumetric MRI. Diffusion metrics showed abnormalities in the corpus callosum, indicating white matter changes in the segments subjected to high strain, as predicted by the model. The same segments also exhibited a severity-dependent increase in glia cell density, white matter thinning and reduced neurofilament expression. Linear mixed effects regression analyses showed that mechanical strain and strain rate were significant predictors of in vivo MRI and histology changes. Specifically, strain and strain rate respectively explained 33% and 28% of the reduction in fractional anisotropy, 51% and 29% of the change in neurofilament expression and 51% and 30% of microglia density changes. The work provides evidence that strain and strain rate in the first milliseconds after injury are important factors in determining patterns of glial and axonal injury and serve as experimental validators of our computational model of traumatic brain injury. Our results provide support for the use of this model in understanding the relationship of biomechanics and neuropathology and can guide the development of head protection systems, such as airbags and helmets.
Keywords: controlled cortical impact; diffusion tensor imaging; finite element modelling; quantitative histology; traumatic brain injury.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Bain AC, Meaney DF. Tissue-level thresholds for axonal damage in an experimental model of central nervous system white matter injury. J Biomech Eng 2000; 122: 615–22. - PubMed
-
- Bechmann I, Nitsch R. Astrocytes and microglial cells incorporate degenerating fibers following entorhinal lesion: a light, confocal, and electron microscopical study using a phagocytosis-dependent labeling technique. Glia 1997; 20: 145–54. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

