Multiorgan Crystal Deposition of an Amphoteric Drug in Rats Due to Lysosomal Accumulation and Conversion to a Poorly Soluble Hydrochloride Salt
- PMID: 33454789
- PMCID: PMC8041455
- DOI: 10.1093/toxsci/kfaa191
Multiorgan Crystal Deposition of an Amphoteric Drug in Rats Due to Lysosomal Accumulation and Conversion to a Poorly Soluble Hydrochloride Salt
Abstract
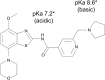
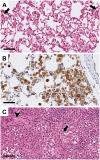
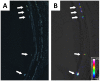
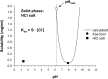
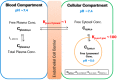
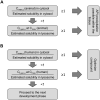
Poor solubility of drug candidates mainly affects bioavailability, but poor solubility of drugs and metabolites can also lead to precipitation within tissues, particularly when high doses are tested. RO0728617 is an amphoteric compound bearing basic and acidic moieties that has previously demonstrated good solubility at physiological pH but underwent widespread crystal deposition in multiple tissues in rat toxicity studies. The aim of our investigation was to better characterize these findings and their underlying mechanism(s), and to identify possible screening methods in the drug development process. Main microscopic features observed in rat RO0728617 toxicity studies were extensive infiltrates of crystal-containing macrophages in multiple organs. Matrix-assisted laser desorption/ionization Fourier transform ion cyclotron resonance mass spectrometry revealed that these crystals contained the orally administered parent compound, and locality was confirmed to be intracytoplasmic and partly intralysosomal by electron microscopic examination. Crystal formation was explained by lysosomal accumulation of the compound followed by precipitation of the hydrochloride salt under physiological conditions in the lysosomes, which have a lower pH and higher chloride concentration in comparison to the cytosol. This study demonstrates that risk of drug precipitation can be assessed by comparing the estimated lysosomal drug concentration at a given dose with the solubility of the compound at lysosomal conditions.
Keywords: crystal deposition; drug precipitation; lysosomal accumulation; macrophages; organ toxicity.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society of Toxicology.
Figures




References
-
- A, ziz R., Helms W. (2009). Pharmacology/toxicology review and evaluation: Pazopanib. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022465s000_Pharm....
-
- Bhatnagar V., Anjaiah S., Puri N., Darshanam B., Ramaiah A. (1993). pH of melanosomes of B 16 murine melanoma is acidic: Its physiological importance in the regulation of melanin biosynthesis. Arch. Biochem. Biophys. 307, 183–192. - PubMed
-
- Boyd B. J., Bergström C. A. S., Vinarov Z., Kuentz M., Brouwers J., Augustijns P., Brandl M., Bernkop-Schnürch A., Shrestha N., Préat V., et al. (2019). Successful oral delivery of poorly water-soluble drugs both depends on the intraluminal behavior of drugs and of appropriate advanced drug delivery systems. Eur. J. Pharm. Sci. 137, 104967. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

