Characterization and comparison of innate and adaptive immune responses at vaccine sites in melanoma vaccine clinical trials
- PMID: 33454795
- PMCID: PMC10992166
- DOI: 10.1007/s00262-020-02844-w
Characterization and comparison of innate and adaptive immune responses at vaccine sites in melanoma vaccine clinical trials
Abstract
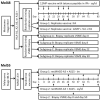
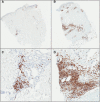
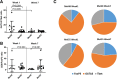
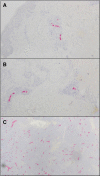
The strength and durability of systemic anti-tumor immune responses induced by cancer vaccines depends on adjuvants to support an immunogenic vaccine site microenvironment (VSME). Adjuvants include water-in-oil emulsions with incomplete Freund's adjuvant (IFA) and combinations of toll-like receptor (TLR) agonists, including a preparation containing TLR4 and TLR9 agonists with QS-21 (AS15). IFA-containing vaccines can promote immune cell accumulation at the VSME, whereas effects of AS15 are largely unexplored. Therefore, we assessed innate and adaptive immune cell accumulation and gene expression at the VSME after vaccination with AS15 and compared to effects with IFA. We hypothesized that AS15 would promote less accumulation of innate and adaptive immune cells at the VSME than IFA vaccines. In two clinical trials, patients with resected high-risk melanoma received either a multipeptide vaccine with IFA or a recombinant MAGE-A3 protein vaccine with AS15. Vaccine site biopsies were obtained after one or multiple vaccines. T cells accumulated early after vaccines with AS15, but this was not durable or of the same magnitude as vaccination in IFA. Vaccines with AS15 increased durable expression of DC- and T cell-related genes, as well as PD-L1 and IDO1, suggesting complex activation and regulation of innate and adaptive immune function with AS15. These changes were generally greater with vaccines containing IFA, but IFA induced reduction in myeloid suppressor cells markers. Evidence of tertiary lymphoid structure (TLS) formation was observed with both adjuvants. Our findings highlight adjuvant-dependent changes in immune features at the VSME that may impact systemic immune responses.
Keywords: Cancer vaccine adjuvant; Clinical trial; Immune response; Melanoma; Tertiary lymphoid structure; Vaccine site.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH, DE part of Springer Nature.
Conflict of interest statement
C.L. Slingluff Jr. reports receiving commercial research grants from Merck, GlaxoSmithKline, 3 M, and Celldex; has ownership interest (including patents) in several of the peptides used in the 12MP vaccine with UVA Licensing and Ventures Group; and is a consultant/advisory board member for Immatics, Curevac, and Polynoma. No potential conflicts of interest were disclosed by the other authors.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

