Durability of hepatitis B surface antigen seroclearance and subsequent risk for hepatocellular carcinoma: A meta-analysis
- PMID: 33455067
- PMCID: PMC7986681
- DOI: 10.1111/jvh.13471
Durability of hepatitis B surface antigen seroclearance and subsequent risk for hepatocellular carcinoma: A meta-analysis
Abstract
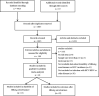
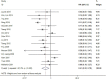
Hepatitis B surface antigen (HBsAg) seroclearance is regarded as the ideal endpoint for antiviral treatment. However, reports on the durability of and outcomes after HBsAg seroclearance are few, which has become a focus in clinical practice. This meta-analysis was performed to evaluate the durability and hepatocellular carcinoma (HCC) incidence after HBsAg seroclearance after treatment cessation. We searched PubMed, Embase, Medline and Web of Science for studies that reported the durability and HCC incidence after HBsAg seroclearance published between 1 January 2000 and 31 January 2020. Data were analysed by a random-effects model. Thirty-eight studies and 43,924 patients were finally included. The results showed that HBsAg seroclearance was durable, with a pooled recurrence rate of 6.19% (95% CI: 4.10%-8.68%). There was no significant difference in recurrence rates after different seroclearance methods or among recurrence types and different regions. Anti-HBs seroconversion resulted in a significantly reduced recurrence rate (RR = 0.25, p < .001). Patients who experienced HBsAg seroclearance had significantly lower HCC incidence than HBsAg-positive (RR = 0.41, p < .001). The pooled HCC incidence after HBsAg seroclearance was 1.88%; this rate was reduced to 0.76% among patients without baseline cirrhosis. In conclusion, the analysis during an average follow-up of 4.74 years suggested that in patients who experienced sustained HBsAg seroclearance and anti-HBs seroconversion, this was associated with low HCC incidence. Patients without baseline cirrhosis benefited even more. We emphasize the importance of gaining HBsAg seroclearance while highlighting the benefits of achieving this as early as possible.
Keywords: HBV; HBsAg; HCC; meta-analysis; recurrence rate.
© 2021 The Authors. Journal of Viral Hepatitis published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373(9663):582‐592. - PubMed
-
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu ; European Association for the Study of the Liver . EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370‐398. - PubMed
-
- Wells G, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta‐analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed February 2, 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

