Evaluation of near point-of-care viral load implementation in public health facilities across seven countries in sub-Saharan Africa
- PMID: 33455081
- PMCID: PMC7811577
- DOI: 10.1002/jia2.25663
Evaluation of near point-of-care viral load implementation in public health facilities across seven countries in sub-Saharan Africa
Abstract
Introduction: In many low- and middle-income countries, HIV viral load (VL) testing occurs at centralized laboratories and time-to-result-delivery is lengthy, preventing timely monitoring of HIV treatment adherence. Near point-of-care (POC) devices, which are placed within health facility laboratories rather than clinics themselves (i.e. "true" POC), can offer VL in conjunction with centralized laboratories to expedite clinical decision making and improve outcomes, especially for patients at high risk of treatment failure. We assessed impacts of near-POC VL testing on result receipt and clinical action in public sector programmes in Cameroon, Democratic Republic of Congo, Kenya, Malawi, Senegal, Tanzania and Zimbabwe.
Methods: Routine health data were collected retrospectively after introducing near-POC VL testing at 57 public sector health facilities (2017 to 2019, country-dependent). Where possible, key indicators were compared to data from patients receiving centralized laboratory testing using hazard ratios and the Somers' D test.
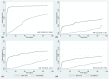
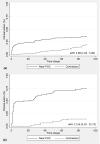
Results: Data were collected from 6795 tests conducted on near-POC and 17614 tests on centralized laboratory-based platforms. Thirty-one percent (2062/6694) of near-POC tests were conducted for high-risk populations: pregnant and breastfeeding women, children and those with suspected failure. Compared to conventional testing, near-POC improved the median time from sample collection to return of results to patient [six vs. sixty-eight days, effect size: -32.2%; 95% CI: -41.0% to -23.4%] and to clinical action for individuals with an elevated HIV VL [three vs. fourty-nine days, effect size: -35.4%; 95% CI: -46.0% to -24.8%]. Near-POC VL results were two times more likely to be returned to the patient within 90 days compared to centralized tests [50% (1781/3594) vs. 27% (4172/15271); aHR: 2.22, 95% CI: 2.05 to 2.39]. Thirty-seven percent (340/925) of patients with an elevated near-POC HIV VL result had documented clinical follow-up actions within 30 days compared to 7% (167/2276) for centralized testing.
Conclusions: Near-POC VL testing enabled rapid test result delivery for high-risk populations and led to significant improvements in the timeliness of patient result receipt compared to centralized testing. While there was some improvement in time-to-clinical action with near-POC VL testing, major gaps remained. Strengthening of systems supporting the utilization of results for patient management are needed to truly capitalize on the benefits of decentralized testing.
Keywords: Africa; point of care; viral load monitoring; viral suppression.
© 2021 The Authors. Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of the International AIDS Society.
Figures
References
-
- WHO . Consolidated guidelines on the use of antiretroviral therapy for treating and preventing HIV infection: recomendations for a public health approach, Seond edn. Geneva: World Health Organization; 2016. - PubMed
-
- Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet. 2014;384(9939):258–71. - PubMed
-
- Quinn TC, Wawer MJ, Sewankambo N,Serwadda D, Li C, Wabwire‐Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;342(13):921–9. - PubMed
-
- HIV Market Report. Clinton Health Access Initiative; 2019.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

