SARS-CoV-2 antigen rapid immunoassay for diagnosis of COVID-19 in the emergency department
- PMID: 33455451
- PMCID: PMC7898296
- DOI: 10.1080/1354750X.2021.1876769
SARS-CoV-2 antigen rapid immunoassay for diagnosis of COVID-19 in the emergency department
Abstract
Background: In the emergency department (ED) setting, rapid testing for SARS-CoV-2 is likely associated with advantages to patients and healthcare workers, for example, enabling early but rationale use of limited isolation resources. Most recently, several SARS-CoV-2 rapid point-of-care antigen tests (AGTEST) became available. There is a growing need for data regarding their clinical utility and performance in the diagnosis of SARS-CoV-2 infection in the real life setting EDs.
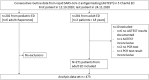
Methods: We implemented AGTEST (here: Roche/SD Biosensor) in all four adult and the one paediatric EDs at Charité - Universitätsmedizin Berlin in our diagnostic testing strategy. Test indication was limited to symptomatic suspected COVID-19 patients. Detailed written instructions on who to test were distributed and testing personnel were trained in proper specimen collection and handling. In each suspected COVID-19 patient, two sequential deep oro-nasopharyngeal swabs were obtained for viral tests. The first swab was collected for nucleic acid testing through SARS-CoV-2 real-time reverse transcriptase (rt)-PCR diagnostic panel (PCRTEST) in the central laboratory. The second swab was collected to perform the AGTEST. Analysis of routine data was prospectively planned and data were retrieved from the medical records after the inclusion period in the adult or paediatric ED. Diagnostic performance was calculated using the PCRTEST as reference standard. False negative and false positive AGTEST results were analysed individually and compared with viral concentrations derived from the calibrated PCRTEST.
Results: We included n = 483 patients including n = 202 from the paediatric ED. N = 10 patients had to be excluded due to missing data and finally n = 473 patients were analysed. In the adult cohort, the sensitivity of the AGTEST was 75.3 (95%CI: 65.8/83.4)% and the specificity was 100 (95%CI: 98.4/100)% with a SARS-CoV-2 prevalence of 32.8%; the positive predictive value was 100 (95%CI: 95.7/100)% and the negative predictive value 89.2 (95%CI: 84.5/93.9)%. In the paediatric cohort, the sensitivity was 72.0 (95%CI: 53.3/86.7)%, the specificity was 99.4 (95%CI:97.3/99.9)% with a prevalence of 12.4%; the positive predictive value was 94.7 (95%CI: 78.3/99.7)% and the negative predictive value was 96.2 (95%CI:92.7/98.3)%. Thus, n = 22 adult and n = 7 paediatric patients showed false negative AGTEST results and only one false positive AGTEST occurred, in the paediatric cohort. Calculated viral concentrations from the rt-PCR lay between 3.16 and 9.51 log10 RNA copies/mL buffer. All false negative patients in the adult ED cohort, who had confirmed symptom onset at least seven days earlier had less than 5 × 105 RNA copies/mL buffer.
Conclusions: We conclude that the use of AGTEST among symptomatic patients in the emergency setting is useful for the early identification of COVID-19, but patients who test negative require confirmation by PCRTEST and must stay isolated until this result becomes available. Adult patients with a false negative AGTEST and symptom onset at least one week earlier have typically a low SARS-CoV-2 RNA concentration and are likely no longer infectious.
Keywords: COVID-19; SARS-CoV-2; emergency department; rapid antigen test; rt-PCR; virus concentration.
Conflict of interest statement
The authors report no conflicts of interest related to this paper.
Figures



Comment in
-
In adults and children in the ED with COVID-19 symptoms or a confirmed contact, a rapid POC antigen test had ≥72% sensitivity and ≥99% specificity vs. RT-PCR.Ann Intern Med. 2021 Jul;174(7):JC83. doi: 10.7326/ACPJ202107200-083. Epub 2021 Jul 6. Ann Intern Med. 2021. PMID: 34224272
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
