Effects of exogenous adiponectin supplementation in early pregnant PCOS mice on the metabolic syndrome of adult female offspring
- PMID: 33455575
- PMCID: PMC7812650
- DOI: 10.1186/s13048-020-00755-z
Effects of exogenous adiponectin supplementation in early pregnant PCOS mice on the metabolic syndrome of adult female offspring
Abstract
Objective: PCOS is a heterogeneous endocrine disorder with both reproductive and metabolic abnormalities. At present, PCOS has been confirmed to have a certain genetic background. Compared with healthy women, the vast majority of PCOS patients have hyperandrogenemia, and this excessive androgen exposure during pregnancy may affect the development of female fetuses. The aim of the current study was to investigate the effect of adiponectin intervention during early pregnancy of obese mice with PCOS on the metabolic phenotype of adult female offspring.
Methods: After the PCOS model was established, C57BL/6J mice were divided into maternal-control, maternal-PCOS, and maternal-PCOS + APN groups. DHEA-induced PCOS mice were supplemented with adiponectin (10 mg/kg/day) in the early pregnancy in order to eliminate adverse hormone exposure and then traced for endocrine indicators in their adult female offspring, which were observed for metabolism syndrome or endocrine disturbance and exhibited the main effects of APN. To further explore the underlying mechanism, the relative expressions of phosphorylated AMPK, PI3K, and Akt were detected in the ovaries of offspring mice.
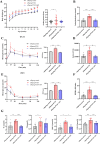
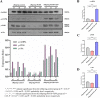
Results: The serum testosterone level of the maternal-PCOS + APN group in early pregnancy was significantly lower than that of the maternal-PCOS group (p < 0.01). The serum testosterone level in the offspring-PCOS + APN group was significantly lower than in the offspring-PCOS group (p <0.05), the diestrus time characterized by massive granulocyte aggregation in the estrus cycle was significantly shorter than in the offspring-PCOS group (p<0.05), and the phenotypes of PCOS-like reproductive disorders and metabolic disorders, such as obesity, insulin resistance, impaired glucose tolerance, and hyperlipidemia, were also significantly improved in the offspring-PCOS + APN group (p < 0.05). Compared with the control group, the expression levels of phosphorylated AMPK, PI3K, and Akt in the offspring-PCOS group were significantly decreased (p < 0.05), while those in the offspring-PCOS + APN group were significantly increased (p < 0.05).
Conclusions: APN intervention in early pregnancy significantly reduced the adverse effects of maternal obesity and high androgen levels during pregnancy on female offspring and corrected the PCOS-like endocrine phenotype and metabolic disorders of adult female offspring. This effect may be caused by the activation of the AMPK/PI3K-Akt signaling pathway in PCOS offspring mice.
Keywords: Adiponectin; Metabolic syndrome; Offspring; Polycystic ovary syndrome.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

