Inhibition of Peptidyl Arginine Deiminase-4 Prevents Renal Ischemia-Reperfusion-Induced Remote Lung Injury
- PMID: 33456369
- PMCID: PMC7787741
- DOI: 10.1155/2020/1724206
Inhibition of Peptidyl Arginine Deiminase-4 Prevents Renal Ischemia-Reperfusion-Induced Remote Lung Injury
Abstract
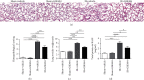
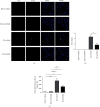
Ischemia reperfusion (IR) can lead to acute kidney injury and can be complicated by acute lung injury, which is one of the leading causes of acute kidney injury-related death. Peptidyl arginine deiminase-4 (PAD4) is a member of the PAD enzyme family and plays a critical role in inflammatory reactions and neutrophil extracellular trap formation in a variety of pathological conditions. It has been reported that PAD4 inhibition can protect certain organs from ischemic injury. In this study, we aimed to understand the mode of action of PAD4 in renal ischemia-reperfusion-mediated acute lung injury. Bilateral renal pedicle occlusion was induced for 30 min followed by reperfusion for 24 h. A specific inhibitor of PAD4, GSK484, was delivered via intraperitoneal injection to alter the PAD4 activity. The pulmonary PAD4 expression, pulmonary impairment, neutrophil infiltration, Cit-H3 expression, neutrophil extracellular trap formation, inflammatory cytokine secretion, and pulmonary apoptosis were analyzed. We found that renal ischemia reperfusion was associated with pulmonary pathological changes and increases in neutrophil infiltration, neutrophil extracellular trap formation, and inflammatory cytokine secretion in the lungs of the recipient animals. Suppression of PAD4 by GSK484 reduced remote lung injury by mitigating neutrophil infiltration, neutrophil extracellular trap formation, apoptosis, and inflammatory factor secretion. Our findings demonstrate that specific PAD4 inhibition by GSK484 may be an effective strategy to attenuate distant lung injury complicating renal ischemia-reperfusion injury.
Copyright © 2020 Mingjun Du et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures





Similar articles
-
Inhibition of peptidyl arginine deiminase-4 protects against myocardial infarction induced cardiac dysfunction.Int Immunopharmacol. 2020 Jan;78:106055. doi: 10.1016/j.intimp.2019.106055. Epub 2019 Dec 6. Int Immunopharmacol. 2020. PMID: 31816575
-
Neutrophil peptidyl arginine deiminase-4 has a pivotal role in ischemia/reperfusion-induced acute kidney injury.Kidney Int. 2018 Feb;93(2):365-374. doi: 10.1016/j.kint.2017.08.014. Epub 2017 Oct 20. Kidney Int. 2018. PMID: 29061334 Free PMC article.
-
Peptidyl arginine deiminase-4 activation exacerbates kidney ischemia-reperfusion injury.Am J Physiol Renal Physiol. 2014 Nov 1;307(9):F1052-62. doi: 10.1152/ajprenal.00243.2014. Epub 2014 Aug 27. Am J Physiol Renal Physiol. 2014. PMID: 25164081 Free PMC article.
-
Inhibition of Neutrophil Extracellular Traps: A Potential Therapeutic Strategy for Hemorrhagic Stroke.J Integr Neurosci. 2025 Apr 23;24(4):26357. doi: 10.31083/JIN26357. J Integr Neurosci. 2025. PMID: 40302254 Review.
-
PAD4 takes charge during neutrophil activation: Impact of PAD4 mediated NET formation on immune-mediated disease.J Thromb Haemost. 2021 Jul;19(7):1607-1617. doi: 10.1111/jth.15313. Epub 2021 May 12. J Thromb Haemost. 2021. PMID: 33773016 Free PMC article. Review.
Cited by
-
Innate Immune Response in SARS-CoV-2 Infection.Microorganisms. 2022 Feb 23;10(3):501. doi: 10.3390/microorganisms10030501. Microorganisms. 2022. PMID: 35336077 Free PMC article.
-
Activation of GPR81 aggravated intestinal ischemia/reperfusion injury-induced acute lung injury via HMGB1-mediated neutrophil extracellular traps formation.Int J Immunopathol Pharmacol. 2023 Jan-Dec;37:3946320231193832. doi: 10.1177/03946320231193832. Int J Immunopathol Pharmacol. 2023. PMID: 37698122 Free PMC article.
-
RGD Peptide and PAD4 Inhibitor-Loaded Gold Nanorods for Chemo-Photothermal Combined Therapy to Inhibit Tumor Growth, Prevent Lung Metastasis and Improve Biosafety.Int J Nanomedicine. 2021 Aug 16;16:5565-5580. doi: 10.2147/IJN.S319210. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34429600 Free PMC article.
-
Platelet factor 4 limits neutrophil extracellular trap- and cell-free DNA-induced thrombogenicity and endothelial injury.JCI Insight. 2023 Nov 22;8(22):e171054. doi: 10.1172/jci.insight.171054. JCI Insight. 2023. PMID: 37991024 Free PMC article.
-
Protein citrullination: inhibition, identification and insertion.Philos Trans R Soc Lond B Biol Sci. 2023 Nov 20;378(1890):20220240. doi: 10.1098/rstb.2022.0240. Epub 2023 Oct 2. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 37778377 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

