Establishment and Validation of a Liquid Chromatography-Tandem Mass Spectrometry Method for the Determination of Tigecycline in Critically Ill Patients
- PMID: 33456470
- PMCID: PMC7785388
- DOI: 10.1155/2020/6671392
Establishment and Validation of a Liquid Chromatography-Tandem Mass Spectrometry Method for the Determination of Tigecycline in Critically Ill Patients
Abstract
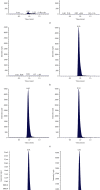
Utilizing tigecycline-d9 as an internal standard (IS), we establish and validate a simple, effective, and rapid liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for the quantitative measurement of tigecycline (TGC) in patient plasma. Acetonitrile was used as a precipitant to process plasma samples by a protein precipitation method. The analyte and IS were separated on an HSS T3 (2.1 × 100 mm, 3.5 μm) chromatographic column using isocratic program with a mobile phase comprising of 80% solvent A (water containing 0.1% formic acid (v/v) with 5 mM ammonium acetate) and 20% solvent B (acetonitrile) with a flow rate of 0.3 mL/min. The mass spectrometer, scanning in multireaction monitoring (MRM) mode and using an electrospray ion source (ESI), operated in the positive-ion mode. The ion pairs used for quantitative analysis were m/z 586.4 ⟶ 513.3 and m/z 595.5 ⟶ 514.3 for TGC and the IS, respectively. The range of the linear calibration curve obtained with this approach was 50-5000 ng/ml. Intra- and interbatch precision for TGC quantitation were less than 7.2%, and the accuracy ranged from 93.4 to 101.8%. The IS-normalized matrix effect was 87 to 104%. Due to its high precision and accuracy, this novel method allows for fast quantitation of TGC with a total analysis time of 2 min. This approach was effectively applied to study the pharmacokinetics of TGC in critically ill adult patients.
Copyright © 2020 Fen Yao et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this article.
Figures

References
-
- Lu Z. Y., Xu N., He B. L., et al. Inhibition of autophagy enhances the selective anti-cancer activity of tigecycline to overcome drug resistance in the treatment of chronic myeloid leukemia. Journal of Experimental & Clinical Cancer Research. 2017;36 doi: 10.1186/s13046-017-0512-6. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

