In Vitro Induction of Pluripotency from Equine Fibroblasts in 20% or 5% Oxygen
- PMID: 33456472
- PMCID: PMC7785345
- DOI: 10.1155/2020/8814989
In Vitro Induction of Pluripotency from Equine Fibroblasts in 20% or 5% Oxygen
Abstract
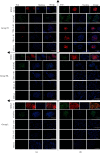
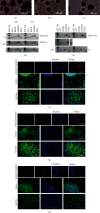
The cellular reprogramming into pluripotency is influenced by external and internal cellular factors, such as in vitro culture conditions (e.g., environmental oxygen concentration), and the aging process. Herein, we aimed to generate and maintain equine iPSCs (eiPSCs) derived from fibroblasts of a horse older than 20 years and to evaluate the effect of different levels of oxygen tension (atmospheric 20% O2, 5% O2, or 20% to 5% O2) on these cells. Fibroblasts were reprogrammed, and putative eiPSCs were positive for positive alkaline phosphatase detection; they were positive for pluripotency-related genes OCT4, REX1, and NANOG; immunofluorescence-positive staining was presented for OCT4 and NANOG (all groups), SOX2 (groups 5% O2 and 20% to 5% O2), and TRA-1-60, TRA-1-81, and SSEA-1 (only in 20% O2); they formed embryoid bodies; and there is spontaneous differentiation in mesoderm, endoderm, and ectoderm embryonic germ layers. In addition to the differences in immunofluorescence analysis results, the eiPSC colonies generated at 20% O2 presented a more compact morphology with a well-defined border than cells cultured in 5% O2 and 20% to 5% O2. Significant differences were also observed in the expression of genes related to glucose metabolism, mitochondrial fission, and hypoxia (GAPDH, GLUT3, MFN1, HIF1α, and HIF2α), after reprogramming. Our results show that the derivation of eiPSCs was not impaired by aging. Additionally, this study is the first to compare high and low oxygen cultures of eiPSCs, showing the generation of pluripotent cells with different profiles. Under the tested conditions, the lower oxygen tension did not favor the pluripotency of eiPSCs. This study shows that the impact of oxygen atmosphere has to be considered when culturing eiPSCs, as this condition influences the pluripotency characteristics.
Copyright © 2020 Raquel V. G. de Castro et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures

Similar articles
-
Cattle In Vitro Induced Pluripotent Stem Cells Generated and Maintained in 5 or 20% Oxygen and Different Supplementation.Cells. 2021 Jun 17;10(6):1531. doi: 10.3390/cells10061531. Cells. 2021. PMID: 34204517 Free PMC article.
-
Generation and miRNA Characterization of Equine Induced Pluripotent Stem Cells Derived from Fetal and Adult Multipotent Tissues.Stem Cells Int. 2019 May 2;2019:1393791. doi: 10.1155/2019/1393791. eCollection 2019. Stem Cells Int. 2019. PMID: 31191664 Free PMC article.
-
Effect of low oxygen tension on transcriptional factor OCT4 and SOX2 expression in New Zealand rabbit bone marrow-derived mesenchymal stem cells.Vet World. 2020 Nov;13(11):2469-2476. doi: 10.14202/vetworld.2020.2469-2476. Epub 2020 Nov 18. Vet World. 2020. PMID: 33363343 Free PMC article.
-
The modification of mitochondrial energy metabolism and histone of goat somatic cells under small molecules compounds induction.Reprod Domest Anim. 2019 Feb;54(2):138-149. doi: 10.1111/rda.13304. Epub 2019 Feb 4. Reprod Domest Anim. 2019. PMID: 30098220
-
Generation of two induced pluripotent stem cells lines from a Mucopolysaccharydosis IIIB (MPSIIIB) patient.Stem Cell Res. 2018 Dec;33:180-184. doi: 10.1016/j.scr.2018.10.019. Epub 2018 Nov 1. Stem Cell Res. 2018. PMID: 30408744
Cited by
-
Cattle In Vitro Induced Pluripotent Stem Cells Generated and Maintained in 5 or 20% Oxygen and Different Supplementation.Cells. 2021 Jun 17;10(6):1531. doi: 10.3390/cells10061531. Cells. 2021. PMID: 34204517 Free PMC article.
-
In vitro induced pluripotency from urine-derived cells in porcine.World J Stem Cells. 2022 Mar 26;14(3):231-244. doi: 10.4252/wjsc.v14.i3.231. World J Stem Cells. 2022. PMID: 35432738 Free PMC article.
-
Influence of Cell Type in In Vitro Induced Reprogramming in Cattle.Life (Basel). 2022 Jul 28;12(8):1139. doi: 10.3390/life12081139. Life (Basel). 2022. PMID: 36013318 Free PMC article.
-
Footprint-free induced pluripotent stem cells can be successfully differentiated into mesenchymal stromal cells in the feline model.Stem Cell Res Ther. 2025 Apr 20;16(1):195. doi: 10.1186/s13287-025-04325-2. Stem Cell Res Ther. 2025. PMID: 40254569 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

