Neutrophil-derived trail is a proinflammatory subtype of neutrophil-derived extracellular vesicles
- PMID: 33456572
- PMCID: PMC7806483
- DOI: 10.7150/thno.51756
Neutrophil-derived trail is a proinflammatory subtype of neutrophil-derived extracellular vesicles
Abstract
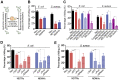
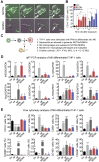
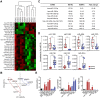
Aims: Extracellular vesicles (EVs) are membrane-derived vesicles that mediate intercellular communications. Neutrophils produce different subtypes of EVs during inflammatory responses. Neutrophil-derived trails (NDTRs) are generated by neutrophils migrating toward inflammatory foci, whereas neutrophil-derived microvesicles (NDMVs) are thought to be generated by neutrophils that have arrived at the inflammatory foci. However, the physical and functional characteristics of neutrophil-derived EVs are incompletely understood. In this study, we aimed to investigate the differences between NDTRs and NDMVs. Methods: The generation of neutrophil-derived EVs were visualized by live-cell fluorescence images and the physical characteristics were further analyzed using nanotracking analysis assay, scanning electron microscopic analysis, and marker expressions. Functional characteristics of neutrophil-derived EVs were analyzed using assays for bactericidal activity, monocyte chemotaxis, phenotype polarization of macrophages, and miRNA sequencing. Finally, the effects of neutrophil-derived EVs on the acute and chronic inflammation were examined in vivo. Results: Both EVs share similar characteristics including stimulators, surface marker expression, bactericidal activity, and chemoattractive effect on monocytes via MCP-1. However, the integrin-mediated physical interaction was required for generation of NDTRs whereas NDMV generation was dependent on PI3K pathway. Interestingly, NDTRs contained proinflammatory miRNAs such as miR-1260, miR-1285, miR-4454, and miR-7975, while NDMVs contained anti-inflammatory miRNAs such as miR-126, miR-150, and miR-451a. Although both EVs were easily uptaken by monocytes, NDTRs enhanced proinflammatory macrophage polarization whereas NDMVs induced anti-inflammatory macrophage polarization. Moreover, NDTRs showed protective effects against lethality in a murine sepsis model and pathological changes in a murine chronic colitis model. Conclusion: These results suggest that NDTR is a proinflammatory subtype of neutrophil-derived EVs distinguished from NDMV.
Keywords: EV, extracellular vesicle; NDMV, neutrophil-derived microvesicle; NDTR, neutrophil-derived trail.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. - PubMed
-
- van der Pol E, Boing AN, Gool EL, Nieuwland R. Recent developments in the nomenclature, presence, isolation, detection and clinical impact of extracellular vesicles. J Thromb Haemost. 2016;14:48–56. - PubMed
-
- Gasser O, Hess C, Miot S, Deon C, Sanchez JC, Schifferli JA. Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp Cell Res. 2003;285:243–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

