Tumor microenvironment remodeling and tumor therapy based on M2-like tumor associated macrophage-targeting nano-complexes
- PMID: 33456579
- PMCID: PMC7806477
- DOI: 10.7150/thno.50928
Tumor microenvironment remodeling and tumor therapy based on M2-like tumor associated macrophage-targeting nano-complexes
Abstract
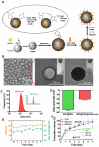
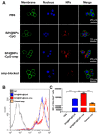
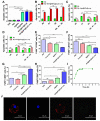
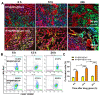
Background: Among the many immunosuppressive cells in the tumor microenvironment, tumor-associated-macrophages (TAMs) are well known to contribute to tumor development. TAMs can be conditioned (polarized) to transition between classical M1-like macrophages, or alternatively to M2-like macrophages. Both are regulated by signaling molecules in the microenvironment. M1-like TAMs can secrete classic inflammatory cytokines that kill tumors by promoting tumor cell necrosis and immune cell infiltration into the tumor microenvironment. In contrast, M2-like TAMs exhibit powerful tumor-promoting functions, including degradation of tumor extracellular matrix, destruction of basement membrane, promotion of angiogenesis, and recruitment of immunosuppressor cells, all of which further promote tumor progression and distal metastasis. Therefore, remodeling the tumor microenvironment by reversing the TAM phenotype will be favorable for tumor therapy, especially immunotherapy. Methods: PLGA nanoparticles encapsulating baicalin and melanoma antigen Hgp peptide fragment 25-33 were fabricated using the ultrasonic double-emulsion technique. The nanoparticles were further loaded with CpG fragments and used conjugated M2pep and α-pep peptides on their surfaces to produce novel nano-complexes. The capability to target M2-like TAMs and anti-tumor immunotherapy effects of nano-complexes were evaluated by flow cytometry and confocal microscopy in vitro. We also investigated the survival and histopathology of murine melanoma models administrated with different nanocomplexes. Improvements in the tumor microenvironment for immune attack of melanoma-bearing mice were also assessed. Results: The nano-complexes were effectively ingested by M2-like TAMs in vitro and in vivo, and the acidic lysosomal environment triggered the disintegration of polydopamine from the nanoparticle surface, which resulted in the release of the payloads. The released CpG played an important role in transforming the M2-like TAMs into the M1-like phenotype that further secreted inflammatory cytokines. The reversal of TAM released cytokines and gradually suppressed tumor angiogenesis, permitting the remodeling of the tumor microenvironment. Moreover, the activated TAMs also presented antigen to T cells, which further stimulated the antitumor immune response that inhibited tumor metastasis. Activated T cells released cytokines, which stimulated NK cell infiltration and directly resulted in killing tumor cells. The baicalin released by M1-like TAMs also killed tumor cells. Conclusion: The nano-complexes facilitated baicalin, antigen, and immunostimulant delivery to M2-like TAMs, which polarized and reversed the M2-like TAM phenotype and remodeled the tumor microenvironment to allow killing of tumor cells.
Keywords: anti-tumor therapy; nano-complex; tumor associated macrophage; tumor microenvironment.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

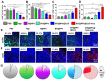
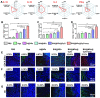
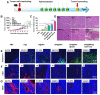
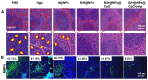
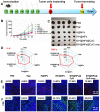
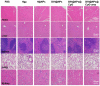
References
-
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F. et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Daneshmandi S, Shahrokhi S. Engineering Tumor Cells with Tumor Necrosis Factor α (TNF-α) or CD40 Ligand (CD40L) Genes Induce Anti-tumor Immune Responses. Int J Pept Res Ther. 2018;25:427–436.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

