Hyaluronic Acid Improves Hydrogen Peroxide Modulatory Effects on Calcium Channel and Sodium-Potassium Pump in 4T1 Breast Cancer Cell Line
- PMID: 33456676
- PMCID: PMC7787766
- DOI: 10.1155/2020/8681349
Hyaluronic Acid Improves Hydrogen Peroxide Modulatory Effects on Calcium Channel and Sodium-Potassium Pump in 4T1 Breast Cancer Cell Line
Abstract
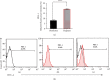
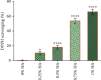
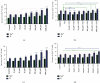
Maintaining homeostasis of ion concentrations is critical in cancer cells. Under hypoxia, the levels of channels and pumps in cancer cells are more active than normal cells suggesting ion channels as a suitable therapeutic target. One of the contemporary ways for cancer therapy is oxidative stress. However, the effective concentration of oxidative stress on tumor cells has been reported to be toxic for normal cells as well. In this study, we benefited from the modifying effects of hyaluronic acid (HA) on H2O2, as a free radical source, to make a gradual release of oxidative stress on cancer cells while preventing/decreasing damage to normal cells under normoxia and hypoxic conditions. To do so, we initially investigated the optimal concentration of HA antioxidant capacity by the DPPH test. In the next step, we found optimum H2O2 dose by treating the 4T1 breast cancer cell line with increasing concentrations (0, 10, 20, 50,100, 200, 500, and 1000 μM) of H2O2 alone or H2O2 + HA (83%) for 24 hrs. The calcium channel and the sodium-potassium pumps were then evaluated by measuring the levels of calcium, sodium, and potassium ions using an atomic absorption flame spectrophotometer. The results revealed that treatment with H2O2 or H2O2+ HA led to an intracellular increase of calcium, sodium, and potassium in the normoxic and hypoxic circumstances in a dose-dependent manner. It is noteworthy that H2O2 + HA treatment had more favorable and controllable effects compared with H2O2 alone. Moreover, HA optimizes the antitumor effect of oxidative stress exerted by H2O2 making H2O2 + HA suitable for clinical use in cancer treatment along with chemotherapy.
Copyright © 2020 Ardeshir Abbasi et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Lasso P., Llano Murcia M., Sandoval T. A., Urueña C., Barreto A., Fiorentino S. Breast tumor cells highly resistant to drugs are controlled only by the immune response induced in an immunocompetent mouse model. Integrative Cancer Therapies. 2019;18 doi: 10.1177/1534735419848047. - DOI - PMC - PubMed
-
- Feng P. Zinc inhibits prostate cancer growth: the American society for cell biology 41st annual meeting. Washington, DC: 2001.
-
- Holcatova I., Bencko V. Environmental epidemiology of malignancies. The central European perspective. Central European journal of public health. 1998;6(1):13–17. - PubMed
-
- Bootman M. D., Rietdorf K., Hardy H., Dautova Y., Corps E., Pierro C., et al. Calcium signalling and regulation of cell function. e LS. 2001
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

