Characterization of FGFR signaling in prostate cancer stem cells and inhibition via TKI treatment
- PMID: 33456711
- PMCID: PMC7800776
- DOI: 10.18632/oncotarget.27859
Characterization of FGFR signaling in prostate cancer stem cells and inhibition via TKI treatment
Abstract
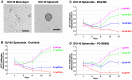
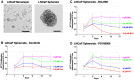
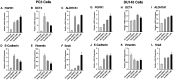
Metastatic castrate-resistant prostate cancer (CRPC) remains uncurable and novel therapies are needed to better treat patients. Aberrant Fibroblast Growth Factor Receptor (FGFR) signaling has been implicated in advanced prostate cancer (PCa), and FGFR1 is suggested to be a promising therapeutic target along with current androgen deprivation therapy. We established a novel in vitro 3D culture system to study endogenous FGFR signaling in a rare subpopulation of prostate cancer stem cells (CSCs) in the cell lines PC3, DU145, LNCaP, and the induced pluripotent iPS87 cell line. 3D-propagation of PCa cells generated spheroids with increased stemness markers ALDH7A1 and OCT4, while inhibition of FGFR signaling by BGJ398 or Dovitinib decreased cell survival and proliferation of 3D spheroids. The 3D spheroids exhibited altered expression of EMT markers associated with metastasis such as E-cadherin, vimentin and Snail, compared to 2D monolayer cells. TKI treatment did not result in significant changes of EMT markers, however, specific inhibition of FGFR signaling by BGJ398 showed more favorable molecular-level changes than treatment with the multi-RTK inhibitor Dovitinib. This study provides evidence for the first time that FGFR1 plays an essential role in the proliferation of PCa CSCs at a molecular and cellular level, and suggests that TKI targeting of FGFR signaling may be a promising strategy for AR-independent CRPC.
Keywords: metastatic castrate-resistant prostate cancer; spheroid culture; tyrosine kinase inhibitor.
Copyright: © 2021 Ko et al.
Conflict of interest statement
CONFLICTS OF INTEREST Authors have no conflicts of interest to declare.
Figures




Similar articles
-
Induction of Neuroendocrine Differentiation in Prostate Cancer Cells by Dovitinib (TKI-258) and its Therapeutic Implications.Transl Oncol. 2017 Jun;10(3):357-366. doi: 10.1016/j.tranon.2017.01.011. Epub 2017 Mar 24. Transl Oncol. 2017. PMID: 28342996 Free PMC article.
-
Down-regulation of E-cadherin enhances prostate cancer chemoresistance via Notch signaling.Chin J Cancer. 2017 Mar 29;36(1):35. doi: 10.1186/s40880-017-0203-x. Chin J Cancer. 2017. PMID: 28356132 Free PMC article.
-
Effects of multi and selective targeted tyrosine kinase inhibitors on function and signaling of different bladder cancer cells.Biomed Pharmacother. 2018 Oct;106:316-325. doi: 10.1016/j.biopha.2018.06.110. Epub 2018 Jul 11. Biomed Pharmacother. 2018. PMID: 29966976
-
FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole-body homeostasis (Review).Int J Mol Med. 2016 Jul;38(1):3-15. doi: 10.3892/ijmm.2016.2620. Epub 2016 May 31. Int J Mol Med. 2016. PMID: 27245147 Free PMC article. Review.
-
ZBTB46, SPDEF, and ETV6: Novel Potential Biomarkers and Therapeutic Targets in Castration-Resistant Prostate Cancer.Int J Mol Sci. 2019 Jun 8;20(11):2802. doi: 10.3390/ijms20112802. Int J Mol Sci. 2019. PMID: 31181727 Free PMC article. Review.
Cited by
-
Comprehensive Analysis of Clinically Relevant Copy Number Alterations (CNAs) Using a 523-Gene Next-Generation Sequencing Panel and NxClinical Software in Solid Tumors.Genes (Basel). 2024 Mar 23;15(4):396. doi: 10.3390/genes15040396. Genes (Basel). 2024. PMID: 38674331 Free PMC article.
-
FGFR1, a novel biomarker for metastatic castration-resistant prostate cancer?Oncotarget. 2021 May 25;12(11):1026-1028. doi: 10.18632/oncotarget.27957. eCollection 2021 May 25. Oncotarget. 2021. PMID: 34084275 Free PMC article. No abstract available.
-
The Multifaceted Role of Aldehyde Dehydrogenases in Prostate Cancer Stem Cells.Cancers (Basel). 2021 Sep 20;13(18):4703. doi: 10.3390/cancers13184703. Cancers (Basel). 2021. PMID: 34572930 Free PMC article. Review.
-
Paradoxical cancer cell proliferation after FGFR inhibition through decreased p21 signaling in FGFR1-amplified breast cancer cells.Breast Cancer Res. 2024 Mar 29;26(1):54. doi: 10.1186/s13058-024-01808-7. Breast Cancer Res. 2024. PMID: 38553760 Free PMC article.
-
Exploring oncology treatment strategies with tyrosine kinase inhibitors through advanced 3D models (Review).Med Int (Lond). 2024 Dec 20;5(2):13. doi: 10.3892/mi.2024.212. eCollection 2025 Mar-Apr. Med Int (Lond). 2024. PMID: 39790707 Free PMC article. Review.
References
-
- Urbanucci A, Sahu B, Seppala J, Larjo A, Latonen LM, Waltering KK, Tammela TL, Vessella RL, Lahdesmaki H, Janne OA, Visakorpi T. Overexpression of androgen receptor enhances the binding of the receptor to the chromatin in prostate cancer. Oncogene. 2012; 31:2153–63. 10.1038/onc.2011.401. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

