Suppressive effects of valproic acid on caudal fin regeneration in adult zebrafish
- PMID: 33456719
- PMCID: PMC7782361
- DOI: 10.1080/19768354.2020.1860126
Suppressive effects of valproic acid on caudal fin regeneration in adult zebrafish
Abstract
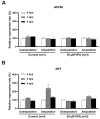
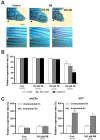
Zebrafish can regenerate fins following injury through an epimorphic process that includes the formation of new tissues and reconstruction of the original morphology. In this study, the effects of valproic acid (VPA), a widely used anti-epileptic drug, on fin regeneration were studied after the caudal fin amputation of adult zebrafish. In the control group, zebrafish formed new tissues and reconstructed the original rays 14 days after amputation (dpa). Meanwhile, VPA treatments between 20 and 200 µM following amputation suppressed fin regeneration in a dose-dependent manner and altered morphological characteristics, such as bifurcation and segmentation, in the rays. Compared to the control, VPA also delayed blastema formation and decreased cell proliferation in the mesenchymal area of the regenerated fin. The mRNA expression of lef1, a downstream signaling gene in the Wnt pathway, was transiently increased in the regenerated fin of the control at 2 dpa; the same increase was not observed in the VPA-treated zebrafish. Sodium butyrate (SB), an histone deacetylase activity (HDAC) inhibitor, suppressed the fin regeneration without affecting the morphological characteristics of the regenerated ray. Furthermore, the transient increase of lef1 mRNA was not suppressed in the SB-treated zebrafish. These results suggested that VPA's suppressive effects on fin regeneration are partly mediated through decreased cell proliferation and lef1 mRNA expression.
Keywords: BrdU; caudal fin; regeneration; valproic acid; zebrafish.
© 2020 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- Becerra J, Montes GS, Bexiga SRR, Junqueira LCU.. 1983. Structure of the tail fin in teleosts. Cell Tissue Res. 230:127–137. - PubMed
-
- Gurvich N, Berman MG, Wittner BS, Gentleman RC, Klein PS, Green JB.. 2005. Association of valproate-induced teratogenesis with histone deacetylase inhibition in vivo. FASEB J. 19(9):1166–1168. - PubMed
-
- Hartmann C. 2007. Skeletal development – Wnts are in control. Mol Cells. 24(2):177–184. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
