Role of magnetic resonance imaging in tumor staging and follow-up for bladder cancer
- PMID: 33457263
- PMCID: PMC7807353
- DOI: 10.21037/tau-19-671
Role of magnetic resonance imaging in tumor staging and follow-up for bladder cancer
Abstract
Urothelial carcinoma of the bladder is a common urologic malignancy. Complex factors, such as local stage, tumor grade, biologic potential, and various conditions, can affect the treatment strategy for bladder cancer. However, the local stage-in particular, the presence or absence of muscle invasion-significantly influences decisions regarding treatment strategy. The role of cystoscopy for screening, diagnosis, and transurethral resection cannot be overlooked. The importance of local staging with magnetic resonance imaging is increasing; magnetic resonance imaging of the bladder is considered a useful staging modality. Moreover, a radiologic reporting system for evaluating and scoring muscle invasion of bladder cancer was recently released. This system is based on multiparametric magnetic resonance imaging and is also expected to be feasible for post-treatment follow-up of bladder cancer. In this review, we discuss the role of magnetic resonance imaging in the local staging of urothelial carcinoma of the urinary bladder and post-treatment imaging. In addition, several technical aspects for obtaining appropriate quality magnetic resonance images of the bladder will be discussed.
Keywords: TNM; Urothelial carcinoma; magnetic resonance imaging; stage; urinary bladder neoplasms.
2020 Translational Andrology and Urology. All rights reserved.
Conflict of interest statement
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-19-671). The series “Muscle-Invasive Bladder Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Figures
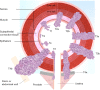
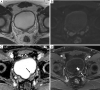

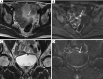
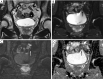
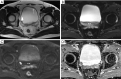
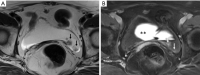
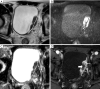

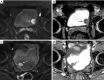
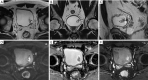
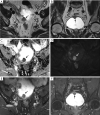
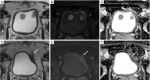
References
-
- Soukup V, Čapoun O, Cohen D, et al. Prognostic performance and reproducibility of the 1973 and 2004/2016 World Health Organization Grading classification systems in non–muscle-invasive bladder cancer: a European Association of Urology non-muscle invasive bladder cancer guidelines panel systematic review. Eur Urol 2017;72:801-13. 10.1016/j.eururo.2017.04.015 - DOI - PubMed
-
- Witjes J, Bruins M, Compérat E, et al. EAU guidelines muscle invasive bladder cancer 2018. EAU Annual Congress Copenhagen, 2018.
Publication types
LinkOut - more resources
Full Text Sources
