Waiting for Godot: A cross sectional survey based analysis of the hydroxychloroquine prophylaxis strategy against COVID-19 in India
- PMID: 33457350
- PMCID: PMC7802012
- DOI: 10.4081/jphr.2020.1888
Waiting for Godot: A cross sectional survey based analysis of the hydroxychloroquine prophylaxis strategy against COVID-19 in India
Abstract
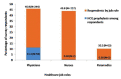
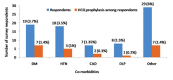
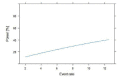
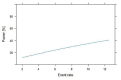
Background: India currently has the second largest burden of infections due to COVID-19. Health Care Worker (HCW) shortages are endemic to Indian healthcare. It should therefore be a huge priority to protect this precious resource as a critical component of the systemic response to this pandemic. Advisories from the Indian Council of Medical Research (ICMR) have focused on using hydroxychloroquine prophylaxis against COVID-19 in at risk HCW. This prophylaxis strategy has no evidence. In further jeopardy there appear to insubstantial attempts to build this evidence as well. In this connection, we commissioned a survey within our Institution to estimate the penetration of hydroxychloroquine (HCQ) use and use this to statistically model the impact of current ongoing studies in India. We also briefly review the literature on HCQ prophylaxis for COVID-19. Design and methods: A structured survey designed using RedCAP application was disseminated among healthcare professionals employed at an academic referral tertiary care centre via online social media platforms. The survey was kept open for the entire month of June 2020. The survey was additionally used to statistically model the size of studies required to comprehensively address the efficacy of HCQ in this setting. Results: 522 responses were received, of which 4 were incomplete. The ICMR strategy of 4 or more doses of HCQ was complete only in 15% of HCW in our survey. The majority of respondents were doctors (238, 46%). Amongst all category of responders, only 12% (n=63) received the full course. A majority of those who initiated the chemoprophylaxis with HCQ turned out to be medical professionals (59/63) with neither nurse nor other categories of healthcare workers accessing the medication. The respondents of our institutional survey did not report any life-threatening side effects. Presuming efficacy as per ICMR modelling for new registry trial on the lines of the published case control study, equal allocation between cases and controls and assuming a RR of 1.3.6, the power of such a study would be very low for n=2000 for event rates from 2.5-12.5%. Conclusion: We report the low penetration of HCQ chemoprophylaxis among the healthcare workers of our institution. We highlight the inherent drawbacks in the study design of current national COVID related trial based on the statistical modelling of our survey results and published literature, and thereby emphasis the need of evidence-based strategies contributing to research policy at national level.
Keywords: COVID trial designs; Hydroxychloroquine chemoprophylaxis; SARS-CoV-2.
©Copyright: the Author(s).
Figures

References
-
- Tanne JH, Hayasaki E, Zastrow M, et al. Covid-19: how doctors and healthcare systems are tackling coronavirus worldwide. BMJ 2020;368:m1090. doi: 10.1136/bmj.m1090 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

